Abstract
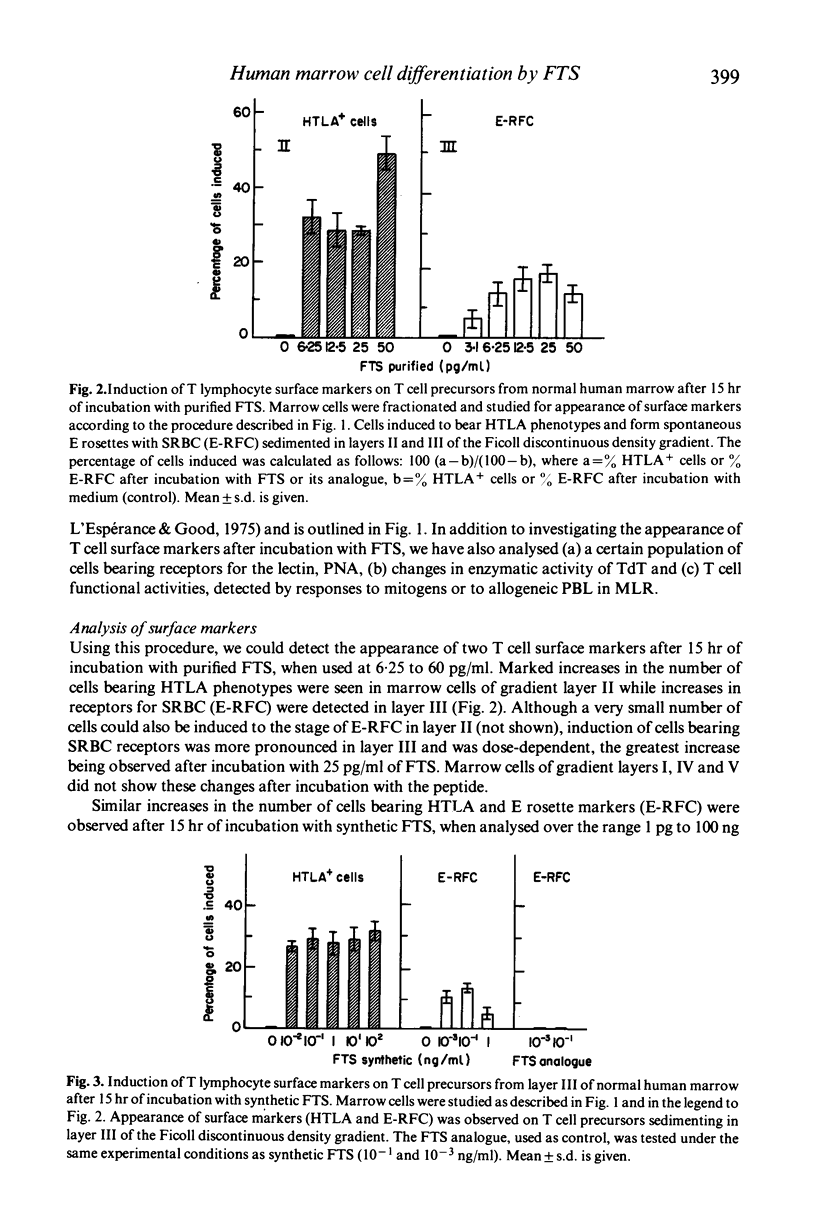
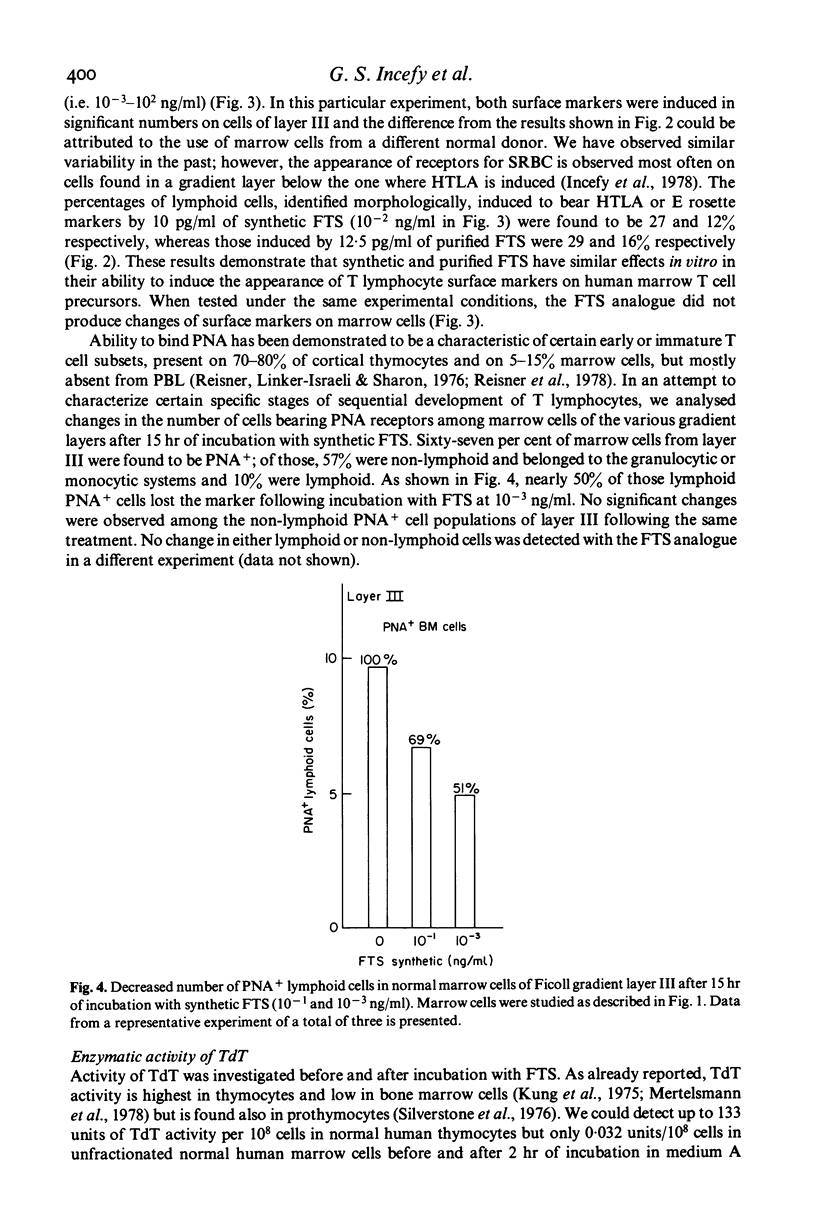
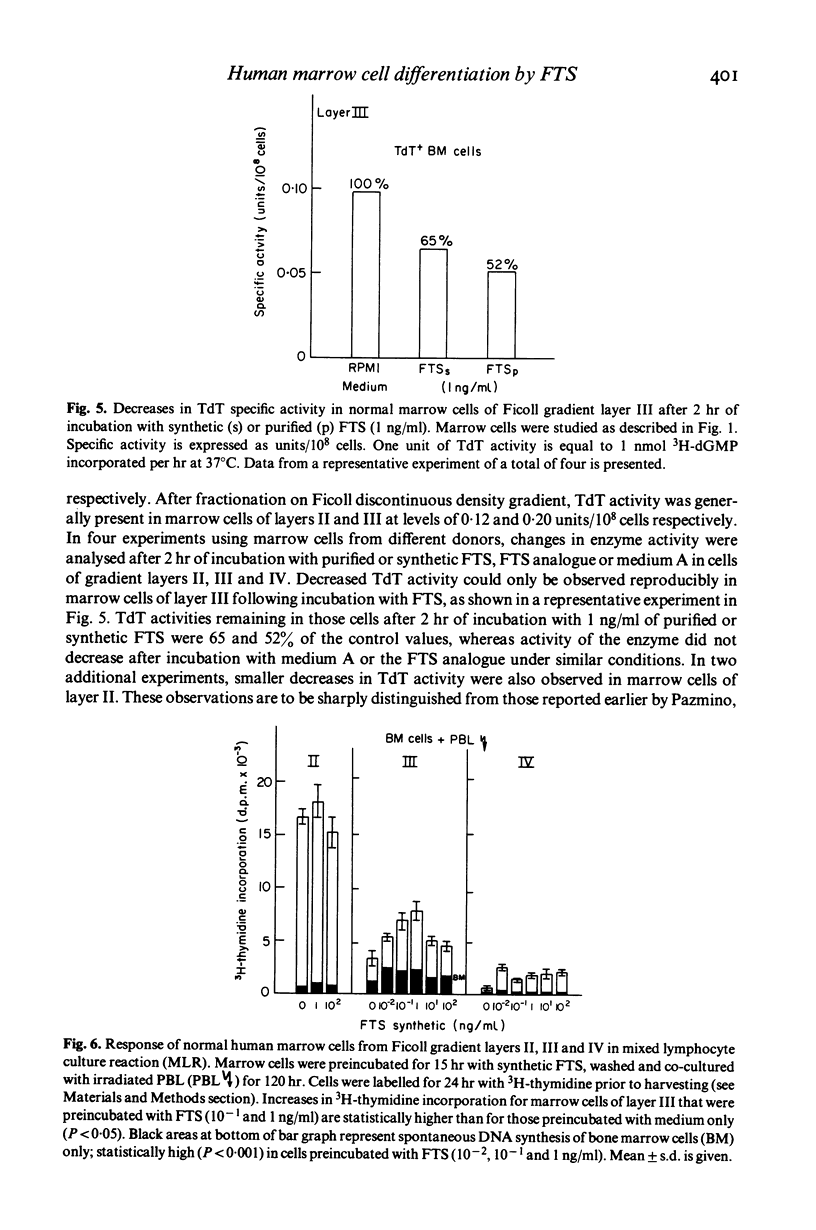
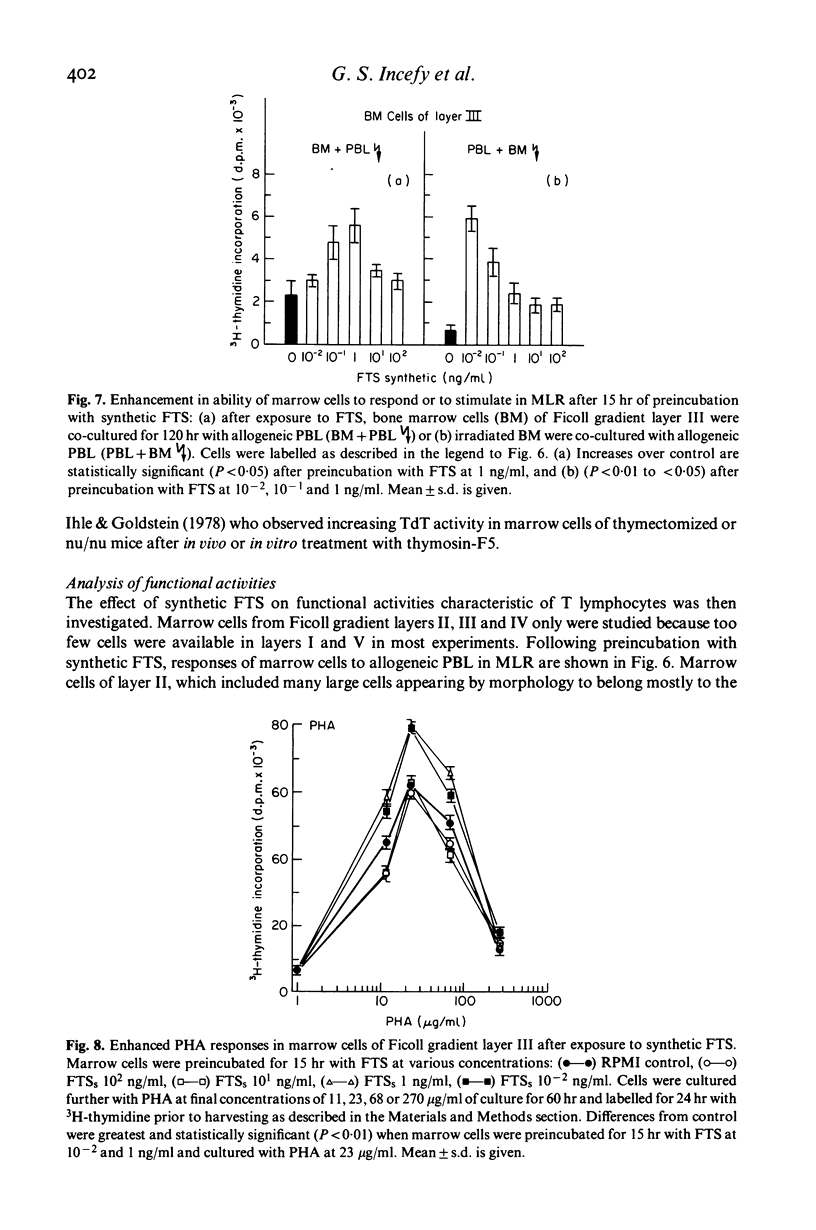
The serum thymic factor, 'facteur thymique serique' (FTS), was analysed in vitro for its ability to induce differentiation of normal human marrow T cell precursors into cells with T lymphocyte characteristics. FTS has been isolated, characterized, sequenced and synthesized. In the mouse, natural and synthetic FTS have similar activities in vitro in the rosette inhibition assay. Both substances influence a variety of T cell differentiation markers and functions in vivo. In this study, we found that synthetic FTS induced appearance of two T cell surface markers, HTLA phenotypes and the ability to form E rosettes, on a selective population of normal human marrow cells sedimenting in layers II or III of a Ficoll discontinuous density gradient. In addition, a population of lymphoid cells also found in layer III, which bears receptors for peanut agglutinin (PNA), was decreased in number following exposure to FTS. In the same gradient layer, cells which expressed terminal deoxyribonucleotidyl transferase (TdT) activity showed decreased activity after treatment with FTS. Functional activities characteristic of T lymphocytes were also enhanced in marrow cells of gradient layer III after preincubation with FTS. These T cell functions were demonstrated in marrow cells by their ability to respond and to stimulate allogeneic peripheral blood lymphocytes (PBL) in mixed lymphocyte reactions and by responses to phytomitogens, PHA, Con A and pokeweed. These changes were not observed in marrow cells of gradient layers I, IV and V or after incubation with an FTS analogue that lacked biological and antigenic activity in the mouse system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Dardenne M., Goldstein A. L., Guha A., White A. Appearance of T-cell markers in bone marrow rosette-forming cells after incubation with thymosin, a thymic hormone. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2734–2738. doi: 10.1073/pnas.68.11.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J., Bardenne M., Pleau J., Rosa J. Biochemical characterisation of a serum thymic factor. Nature. 1977 Mar 3;266(5597):55–57. doi: 10.1038/266055a0. [DOI] [PubMed] [Google Scholar]
- Bach M. A. Lymphocyte-mediated cytotoxicity: effects of ageing, adult thymectomy and thymic factor. J Immunol. 1977 Aug;119(2):641–647. [PubMed] [Google Scholar]
- Dardenne M., Charreire J., Bach J. F. Alterations in thymocyte surface markers after in vivo treatment by serum thymic factor. Cell Immunol. 1978 Aug;39(1):47–54. doi: 10.1016/0008-8749(78)90081-3. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Papiernik M., Bach J. F., Stutman O. Studies on thymus products. 3. Epithelial origin of the serum thymic factor. Immunology. 1974 Aug;27(2):299–304. [PMC free article] [PubMed] [Google Scholar]
- Dardenne M., Pleau J. M., Man N. K., Bach J. F. Structural study of circulating thymic factor: a peptide isolated from pig serum. I. Isolation and purification. J Biol Chem. 1977 Nov 25;252(22):8040–8044. [PubMed] [Google Scholar]
- DuPont B., Hansen J. A. Human mixed-lymphocyte culture reaction: genetics, specificity, and biological implications. Adv Immunol. 1976;23:107–202. doi: 10.1016/s0065-2776(08)60320-x. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Low T. L., McAdoo M., McClure J., Thurman G. B., Rossio J., Lai C. Y., Chang D., Wang S. S., Harvey C. Thymosin alpha1: isolation and sequence analysis of an immunologically active thymic polypeptide. Proc Natl Acad Sci U S A. 1977 Feb;74(2):725–729. doi: 10.1073/pnas.74.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. Isolation of bovine thymin: a polypeptide hormone of the thymus. Nature. 1974 Jan 4;247(5435):11–14. doi: 10.1038/247011a0. [DOI] [PubMed] [Google Scholar]
- Incefy G. S., Boumsell L., Touraine J. L., Espérance P. L., Smithwick E., O'Reilly R., Good R. A. Enhancement of T-lymphocyte differentiation in vitro by thymic extracts after bone marrow transplantation in severe combined immunodeficiencies. Clin Immunol Immunopathol. 1975 Jul;4(2):258–268. doi: 10.1016/0090-1229(75)90061-6. [DOI] [PubMed] [Google Scholar]
- Incefy G. S., Grimes E., Kagan W. A., Goldstein G., Smithwick E., O'Reilly R., Good R. A. Heterogeneity of stem cells in severe combined immunodeficiency. Clin Exp Immunol. 1976 Sep;25(3):462–471. [PMC free article] [PubMed] [Google Scholar]
- Incefy G. S., L'Esperance P., Good R. A. In vitro differentiation of human marrow cells into T lymphocytes by thymic extracts using the rosette technique. Clin Exp Immunol. 1975 Mar;19(3):475–483. [PMC free article] [PubMed] [Google Scholar]
- Komuro K., Boyse E. A. In-vitro demonstration of thymic hormone in the mouse by conversion of precursor cells into lymphocytes. Lancet. 1973 Apr 7;1(7806):740–743. doi: 10.1016/s0140-6736(73)92127-2. [DOI] [PubMed] [Google Scholar]
- Kung P. C., Siverstone A. E., McCaffrey R. P., Baltimore D. Murine terminal deoxynucleotidyl transferase: cellular distribution and response to cortisone. J Exp Med. 1975 Apr 1;141(4):855–865. [PMC free article] [PubMed] [Google Scholar]
- London J., Berrih S., Bach J. F. Peanut agglutinin. I. A new tool for studying T lymphocyte subpopulations. J Immunol. 1978 Aug;121(2):438–443. [PubMed] [Google Scholar]
- Pazmiño N. H., Ihle J. N., Goldstein A. L. Induction in vivo and in vitro of terminal deoxynucleotidyl transferase by thymosin in bone marrow cells from athymic mice. J Exp Med. 1978 Mar 1;147(3):708–718. doi: 10.1084/jem.147.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleau J. M., Dardenne M., Blouquit Y., Bach J. F. Structural study of circulating thymic factor: a peptide isolated from pig serum. II. Amino acid sequence. J Biol Chem. 1977 Nov 25;252(22):8045–8047. [PubMed] [Google Scholar]
- Reisner Y., Itzicovitch L., Meshorer A., Sharon N. Hemopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2933–2936. doi: 10.1073/pnas.75.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Cantor H., Goldstein G., Baltimore D. Terminal deoxynucleotidyl transferase is found in prothymocytes. J Exp Med. 1976 Aug 1;144(2):543–548. doi: 10.1084/jem.144.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touraine J. L., Incefy G. S., Touraine F., Rho Y. M., Good R. A. Differentiation of human bone marrow cells into T lymphocytes by in vitro incubation with thymic extracts. Clin Exp Immunol. 1974 May;17(1):151–158. [PMC free article] [PubMed] [Google Scholar]


