Abstract
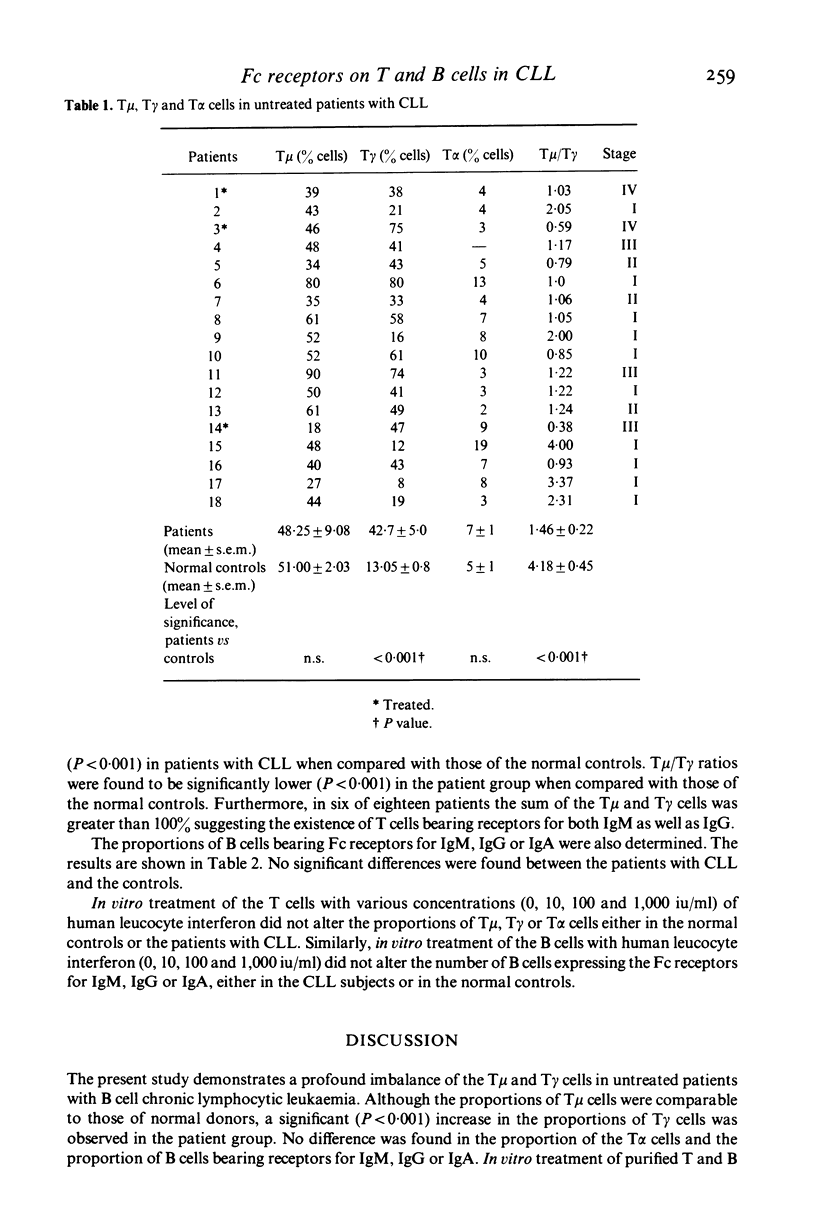
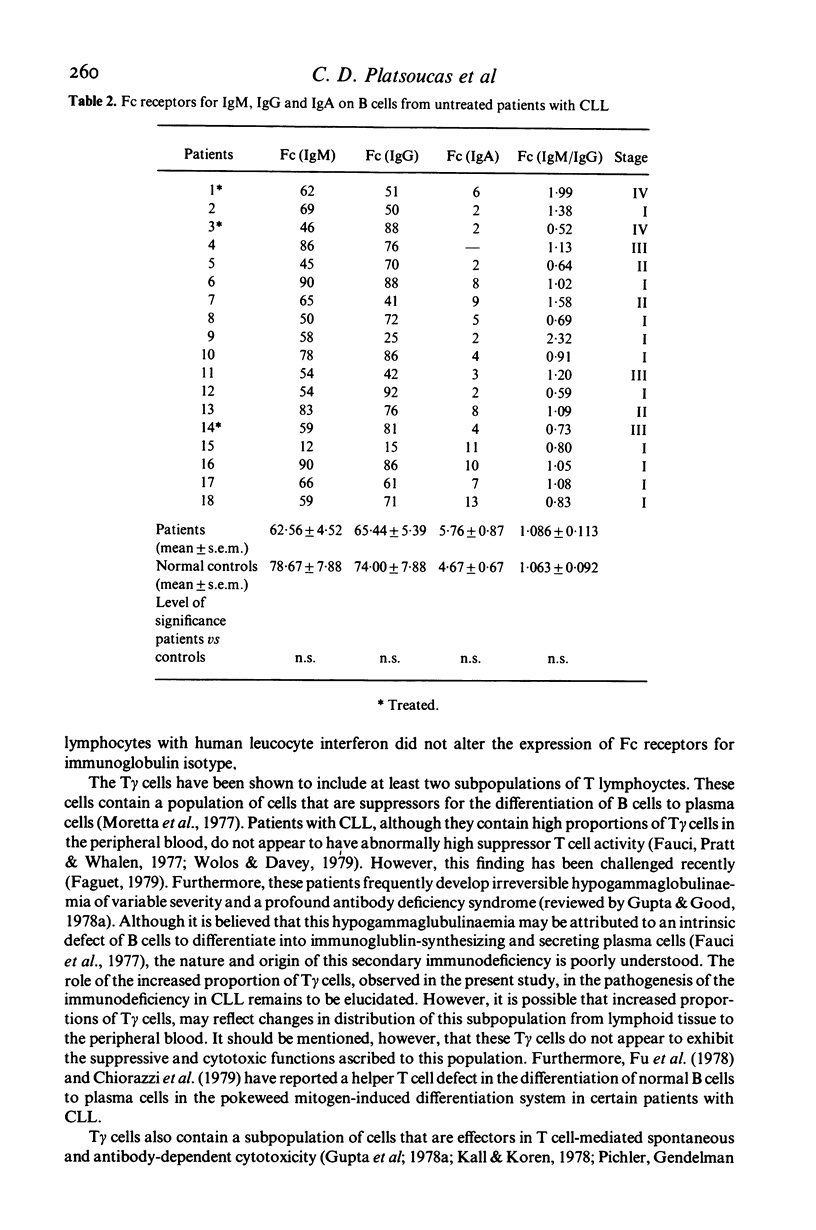
Peripheral blood lymphocytes from eighteen untreated patients with chronic lymphocytic leukaemia (CLL) were analysed for the proportions of T and B lymphocytes with receptors for IgM, IgG or IgA. T lymphocytes with Fc receptors for IgM (T mu cells) or IgA (T alpha) cells were found in proportions comparable to those found in the controls. However, the proportion of T lymphocytes with receptors for IgG (T gamma cells) was significantly increased (P < 0.001) resulting in an abnormally low ratio of T mu/T gamma (P < 0.001), when compared with normal controls. The proportion of B cells bearing Fc receptors for IgM, IgG or IgA was determined simultaneously. No significant differences were found between the normal controls and the patients with CLL. In vitro treatment of the purified T and B lymphocyte preparations with human leucocyte interferon, did not alter the proportions of the lymphocytes expressing Fc receptors for various immunoglobulin isotypes. The significance of these findings is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Bloch K. J. Immunoglobulins on the surface of neoplastic lymphocytes. N Engl J Med. 1972 Aug 10;287(6):272–276. doi: 10.1056/NEJM197208102870603. [DOI] [PubMed] [Google Scholar]
- Catovsky D., Miliani E., Okos A., Galton D. A. Clinical significance of T-cells in chronic lymphocytic leukaemia. Lancet. 1974 Sep 28;2(7883):751–752. doi: 10.1016/s0140-6736(74)90944-1. [DOI] [PubMed] [Google Scholar]
- Chiorazzi N., Fu S. M., Montazeri G., Kunkel H. G., Rai K., Gee T. T cell helper defect in patients with chronic lymphocytic leukemia. J Immunol. 1979 Mar;122(3):1087–1090. [PubMed] [Google Scholar]
- Dao C., Marie J. P., Bernadou A., Bilski-Pasquier G. T-lymphocyte colonies in the lymphoproliferative disorders. Immunology. 1978 Apr;34(4):741–750. [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Adkinson N. F., Jr, Terry W. D. Evidence for individual human peripheral blood lymphocytes bearing both B and T cell markers. Nature. 1974 Jan 25;247(5438):213–215. doi: 10.1038/247213a0. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Siegal F. P., Bentwich Z. H., Kunkel H. G. Lymphocyte binding of aggregated IgG and surface Ig staining in chronic lymphocytic leukaemia. Clin Exp Immunol. 1973 May;14(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Einhorn S., Blomgren H., Strander H. Interferon and spontaneous cytotoxicity in man. I. Enhancement of the spontaneous cytotoxicity of peripheral lymphocytes by human leukocyte interferon. Int J Cancer. 1978 Oct 15;22(4):405–412. doi: 10.1002/ijc.2910220407. [DOI] [PubMed] [Google Scholar]
- Ewan P. W., Lachmann P. J. Demonstration of T-cell and K-cell cytotoxicity against measles-infected cells in normal subjects, multiple sclerosis and subacute sclerosing panencephalitis. Clin Exp Immunol. 1977 Oct;30(1):22–31. [PMC free article] [PubMed] [Google Scholar]
- Faguet G. B. Mechanisms of lymphocyte activation: the role of suppressor cells in the proliferative responses of chronic lymphatic leukemia lymphocytes. J Clin Invest. 1979 Jan;63(1):67–74. doi: 10.1172/JCI109280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M., Hoffman T., Fu S. M., Winchester R., Kunkel H. G. Receptors for IgM on certain human B lymphocytes. J Immunol. 1977 Oct;119(4):1525–1529. [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Foa R., Catovsky D. T-lymphocyte colonies in normal blood, bone marrow and lymphoproliferative disorders. Clin Exp Immunol. 1979 Jun;36(3):488–495. [PMC free article] [PubMed] [Google Scholar]
- Foulis A. K., Cochran A. J., Anderson J. R. Surface immunoglobulins of leukaemic cells. Clin Exp Immunol. 1973 Aug;14(4):481–490. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Molina A., Spiegelberg H. L. A subpopulation of normal human peripheral B lymphcytes that bind IgE. J Clin Invest. 1977 Apr;59(4):616–624. doi: 10.1172/JCI108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Fernandes G., Nair M., Good R. A. Spontaneous and antibody-dependent cell-mediated cytotoxicity by human T cell subpopulations. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5137–5141. doi: 10.1073/pnas.75.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Functionally distinct subpopulations of human T lymphocytes--a review. Clin Bull. 1978;8(3):100–106. [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. II. Effect of thymopoietin, corticosteroids, and irradiation. Cell Immunol. 1977 Nov;34(1):10–18. doi: 10.1016/0008-8749(77)90224-6. [DOI] [PubMed] [Google Scholar]
- Gupta S., Grieco M. H. Rosette formation with mouse erythrocytes: probable marker for human B lymphocytes. Int Arch Allergy Appl Immunol. 1975;49(6):734–742. doi: 10.1159/000231457. [DOI] [PubMed] [Google Scholar]
- Gupta S., Malaviya A. N., Rajagopalan P., Good R. A. Subpopulations of human T lymphocytes. IX. Imbalance of T cell subpopulations in patients with progressive systemic sclerosis. Clin Exp Immunol. 1979 Nov;38(2):342–347. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Platsoucas C. D., Good R. A. Receptors for IgA on a subpopulation of human B lymphocytes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4025–4028. doi: 10.1073/pnas.76.8.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Platsoucas C. D., Schulof R. S., Good R. A. Receptors for IgA on a subpopulation of human T and B lymphocytes. Cell Immunol. 1979 Jul;45(2):469–470. doi: 10.1016/0008-8749(79)90408-8. [DOI] [PubMed] [Google Scholar]
- Gupta S., Safai B., Good R. A. Subpopulations of human T lymphocytes. IV. Quantitation and distribution in patients with mycosis fungoides and Sézary syndrome. Cell Immunol. 1978 Aug;39(1):18–26. doi: 10.1016/0008-8749(78)90078-3. [DOI] [PubMed] [Google Scholar]
- Han T., Dadey B. In vitro functional studies of mononuclear cells in patients with CLL: evidence for functionally normal T lymphocytes and monocytes and abnormal B lymphocytes. Cancer. 1979 Jan;43(1):109–117. doi: 10.1002/1097-0142(197901)43:1<109::aid-cncr2820430117>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Herberman R. R., Ortaldo J. R., Bonnard G. D. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature. 1979 Jan 18;277(5693):221–223. doi: 10.1038/277221a0. [DOI] [PubMed] [Google Scholar]
- Insel R. A., Melewicz F. M., La Via M. F., Balch C. M. Morphology, surface markers, and in vitro responses of a human leukemic T cell. Clin Immunol Immunopathol. 1975 Sep;4(3):382–391. doi: 10.1016/0090-1229(75)90007-0. [DOI] [PubMed] [Google Scholar]
- Kall M. A., Koren H. S. Heterogeneity of human natural killer cell populations. Cell Immunol. 1978 Sep 15;40(1):58–68. doi: 10.1016/0008-8749(78)90315-5. [DOI] [PubMed] [Google Scholar]
- Koziner B., Filippa D. A., Mertelsmann R., Gupta S., Clarkson B., Good R. A., Siegal F. P. Characterization of malignant lymphomas in leukemic phase by multiple differentiation markers of mononuclear cells. Correlations with clinical features and conventional morphology. Am J Med. 1977 Oct;63(4):556–567. doi: 10.1016/0002-9343(77)90201-7. [DOI] [PubMed] [Google Scholar]
- Lum L. G., Muchmore A. V., Keren D., Decker J., Koski I., Strober W., Blaese R. M. A receptor for IgA on human T lymphocytes. J Immunol. 1979 Jan;122(1):65–69. [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Cooper M. D. Characterization of human T-cell subpopulations as defined by specific receptors for immunoglobulins. Contemp Top Immunobiol. 1978;8:19–53. doi: 10.1007/978-1-4684-0922-2_2. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler W. J., Gendelman F. W., Nelson D. L. Fc receptors on human T lymphocytes. II. Cytotoxic capabilities of human T gamma, T mu, B, and L cells. Cell Immunol. 1979 Feb;42(2):410–417. doi: 10.1016/0008-8749(79)90206-5. [DOI] [PubMed] [Google Scholar]
- Pichler W. J., Knapp W. Receptors for IgM on human B lymphocytes. Scand J Immunol. 1978;7(2):105–109. doi: 10.1111/j.1365-3083.1978.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Platsoucas C. D., Good R. A., Gupta S. Separation of human T lymphocyte subpopulations (Tmu, Tgamma) by density gradient electrophoresis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1972–1976. doi: 10.1073/pnas.76.4.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Rabellino E. M., Polley M. J., Grey H. M. Combined studies of complement receptor and surface immunoglobulin-bearing cells and sheep erythrocyte rosette-forming cells in normal and leukemic human lymphocytes. J Clin Invest. 1973 Feb;52(2):377–385. doi: 10.1172/JCI107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Dainer P. M. Fc receptors for IgG, IgM and IgE on human leukaemic lymphocytes. Clin Exp Immunol. 1979 Feb;35(2):286–295. [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos G., Elliott E. V. Formation of mouse or sheep red-blood-cell rosettes by lymphocytes from normal and leukaemic individuals. Lancet. 1974 Apr 6;1(7858):600–601. doi: 10.1016/s0140-6736(74)92655-5. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompeter R. S., Layward L., Hayward A. R. Primary and secondary abnormalities of T cell subpopulations. Clin Exp Immunol. 1978 Dec;34(3):388–392. [PMC free article] [PubMed] [Google Scholar]
- Vignaux F., Gresser I. Differential effects of interferon on the expression of H-2K, H-2D, and Ia antigens on mouse lymphocytes. J Immunol. 1977 Feb;118(2):721–723. [PubMed] [Google Scholar]
- Wolos J. A., Davey F. R. Depressed stimulation in the MLR by B lymphocytes on chronic lymphocytic leukemia: failure to demonstrate a suppressor cell. Clin Immunol Immunopathol. 1979 Sep;14(1):77–85. doi: 10.1016/0090-1229(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Ishizaka K. Lymphocytes bearing Fc receptors for IgE. I. Presence of human and rat T lymphocytes with Fc epsilon receptors. J Immunol. 1979 Jun;122(6):2577–2583. [PubMed] [Google Scholar]
- Yodoi J., Takatsuki K., Masuda T. Letter: Two cases of T-cell chronic lymphocytic leukemia in Japan. N Engl J Med. 1974 Mar 7;290(10):572–573. doi: 10.1056/NEJM197403072901018. [DOI] [PubMed] [Google Scholar]


