Abstract
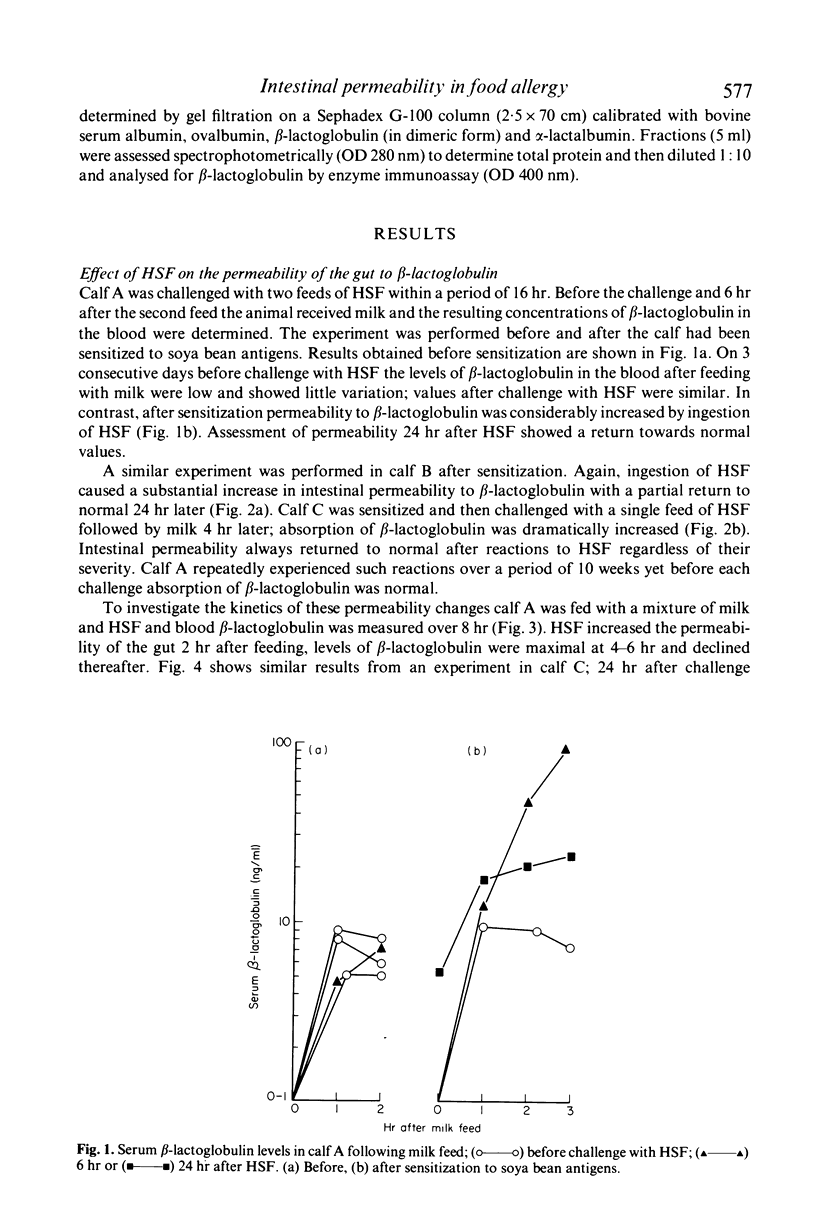
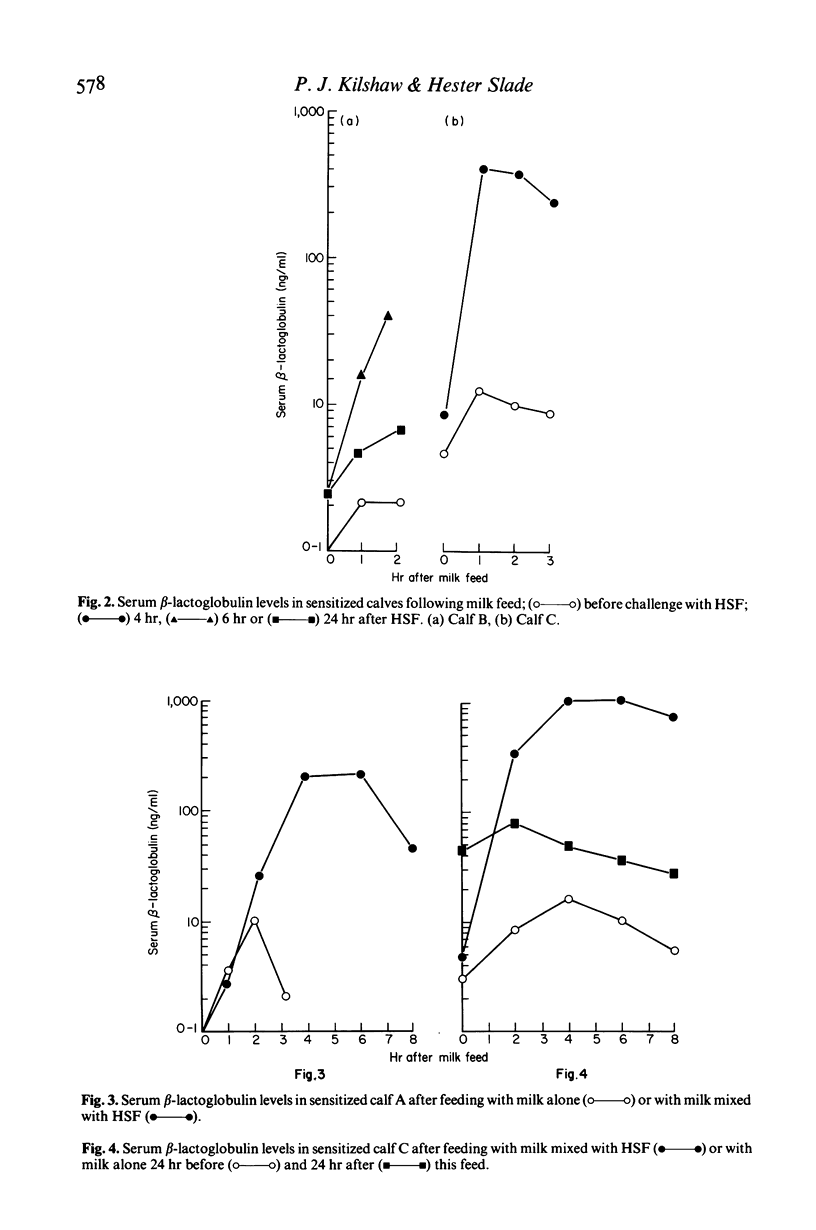
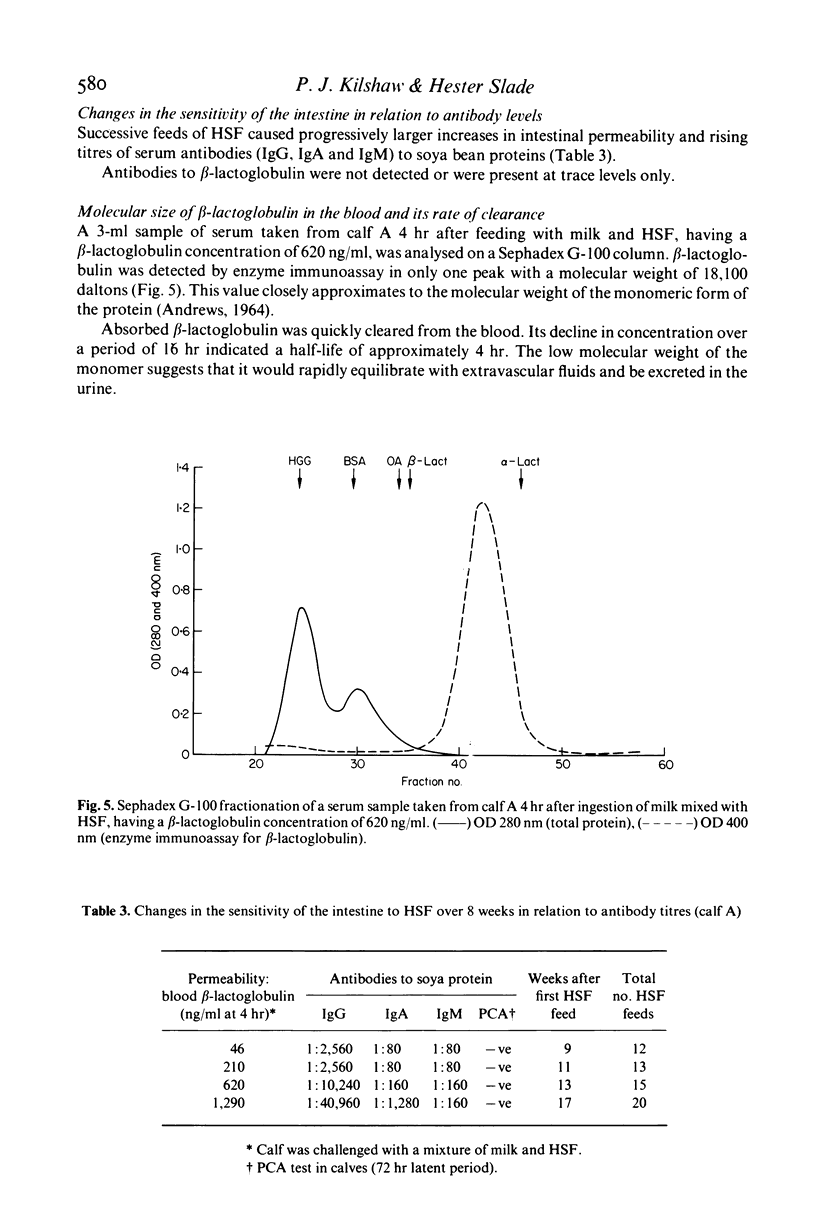
Preruminant calves given a series of feeds of heated soya bean flour (HSF) frequently develop gastrointestinal hypersensitivity to soya bean antigens. The permeability of the intestinal mucosa of calves undergoing hypersensitivity reactions to ingested HSF has been assessed by feeding them milk and quantitating the leakage of beta-lactoglobulin into the blood. Maternal beta-lactoglobulin did not evoke an antibody response in calves. Therefore its detection in the serum was not influenced by immunological mechanisms that normally remove or exclude antigens from the circulation. In sensitized calves ingestion of HSF caused a dramatic increase in the permeability of the intestine to beta-lactoglobulin. The change was transitory and after 24 hr permeability had almost returned to normal. The mucosal barrier was not permanently damaged regardless of the number or the severity of the reactions experienced. Indomethacin was ineffective in counteracting permeability changes. A progressive increase in the sensitivity of the gut to soya bean antigens was accompanied by a rise in the titre of serum antibodies to soya bean proteins. Absorbed beta-lactoglobulin was present in the serum in its monomeric form only, and quickly disappeared from the circulation. In an enzyme immunoassay used to measure its concentration absorbed beta-lactoglobulin was indistinguishable from the native protein. These results suggest that measurement of intestinal permeability to macromolecules might be useful in the diagnosis of certain forms of food allergy in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt M. E., Porter P. Immunoglobulin classes implicated in intestinal disturbances of calves associated with soya protein antigens. J Immunol. 1979 Aug;123(2):676–680. [PubMed] [Google Scholar]
- Beadle G. G., Pay T. W. Reaginic antibody in cattle hypersensitised by foot-and-mouth disease vaccine. Res Vet Sci. 1975 Jul;19(1):1–7. [PubMed] [Google Scholar]
- Bloch K. J., Bloch D. B., Stearns M., Walker W. A. Intestinal uptake of macromolecules. VI. Uptake of protein antigen in vivo in normal rats and in rats infected with Nippostrongylus brasiliensis or subjected to mild systemic anaphylaxis. Gastroenterology. 1979 Nov;77(5):1039–1044. [PubMed] [Google Scholar]
- Brostoff J., Carini C., Wraith D. G., Johns P. Production of IgE complexes by allergen challenge in atopic patients and the effect of sodium cromoglycate. Lancet. 1979 Jun 16;1(8129):1268–1270. doi: 10.1016/s0140-6736(79)92229-3. [DOI] [PubMed] [Google Scholar]
- Buisseret P. D., Youlten L. F., Heinzelmann D. I., Lessof M. H. Prostaglandin-synthesis inhibitors in prophylaxis of food intolerance. Lancet. 1978 Apr 29;1(8070):906–908. doi: 10.1016/s0140-6736(78)90683-9. [DOI] [PubMed] [Google Scholar]
- Burka J. F., Eyre P. A study of prostaglandins and prostaglandin antagonists in relation to anaphylaxis in calves. Can J Physiol Pharmacol. 1974 Oct;52(5):942–951. doi: 10.1139/y74-123. [DOI] [PubMed] [Google Scholar]
- Byars N. E., Ferraresi R. W. Intestinal anaphylaxis in the rat as a model of food allergy. Clin Exp Immunol. 1976 May;24(2):352–356. [PMC free article] [PubMed] [Google Scholar]
- Dannaeus A., Inganäs M., Johansson S. G., Foucard T. Intestinal uptake of ovalbumin in malabsorption and food allergy in relation to serum IgG antibody and orally administered sodium cromoglycate. Clin Allergy. 1979 May;9(3):263–270. doi: 10.1111/j.1365-2222.1979.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Kilshaw P. J., Sissons J. W. Gastrointestinal allergy to soyabean protein in preruminant calves. Allergenic constituents of soyabean products. Res Vet Sci. 1979 Nov;27(3):366–371. [PubMed] [Google Scholar]
- Kilshaw P. J., Sissons J. W. Gastrointestinal allergy to soyabean protein in preruminant calves. Antibody production and digestive disturbances in calves fed heated soyabean flour. Res Vet Sci. 1979 Nov;27(3):361–365. [PubMed] [Google Scholar]
- Kingham J. G., Loehry C. A. Permeability of the small intestine after intra-arterial injection of histamine-type mediators and irradiation. Gut. 1976 Jul;17(7):517–526. doi: 10.1136/gut.17.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganelli R., Levinsky R. J., Brostoff J., Wraith D. G. Immune complexes containing food proteins in normal and atopic subjects after oral challenge and effect of sodium cromoglycate on antigen absorption. Lancet. 1979 Jun 16;1(8129):1270–1272. doi: 10.1016/s0140-6736(79)92230-x. [DOI] [PubMed] [Google Scholar]
- Sissons J. W., Smith R. H. The effect of different diets including those containing soya-bean products, on digesta movement and water and nitrogen absorption in the small intestine of the pre-ruminant calf. Br J Nutr. 1976 Nov;36(3):421–438. doi: 10.1079/bjn19760097. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Walker W. A. Host defense mechanisms in the gastrointestinal tract. Pediatrics. 1976 Jun;57(6):901–916. [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterology. 1974 Sep;67(3):531–550. [PubMed] [Google Scholar]
- Weits J., de Gast G. C., The T. H., Esselink M. T., Deelder A. M., Petrovic M., Mandema E. Class-specific antibody titres (ELISA) against the primary immunogen Helix pomatia haemocyanin (HPH) in man. Clin Exp Immunol. 1978 Jun;32(3):443–450. [PMC free article] [PubMed] [Google Scholar]
- Williams T. J., Peck M. J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]


