Abstract
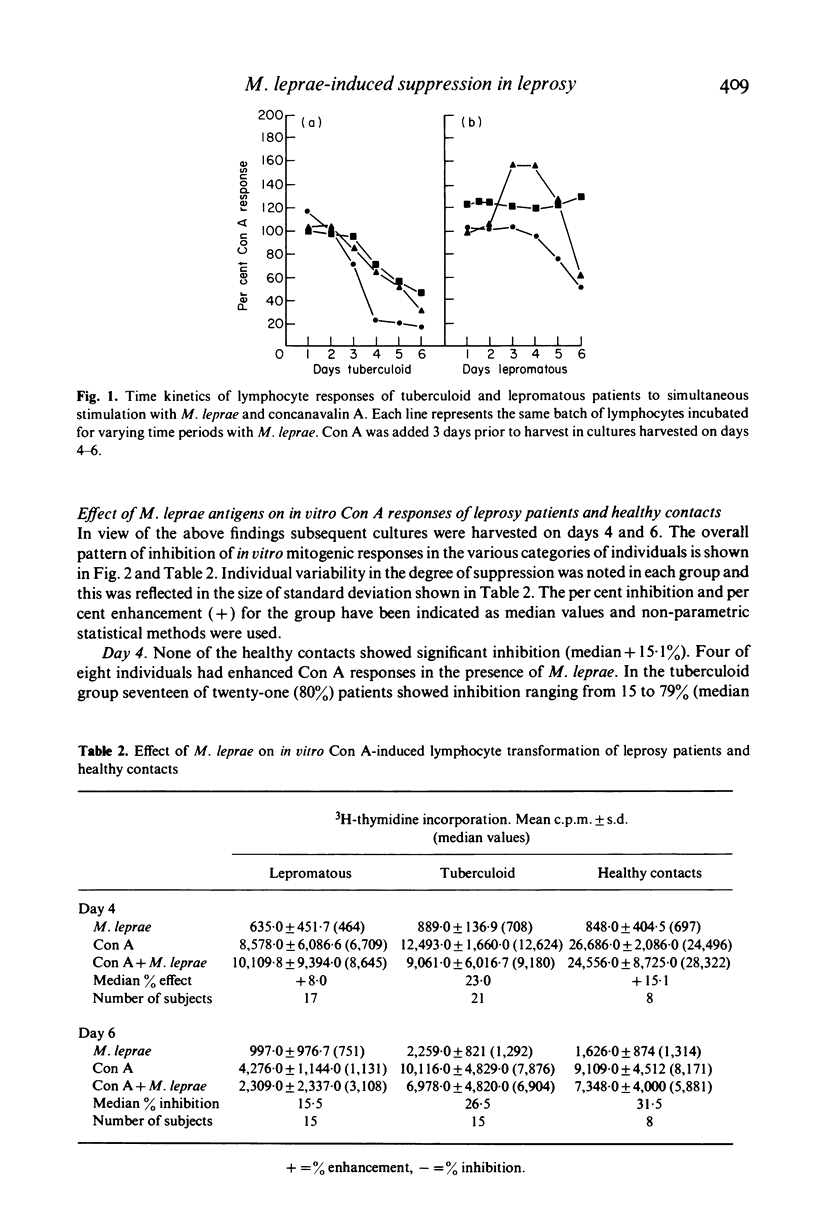
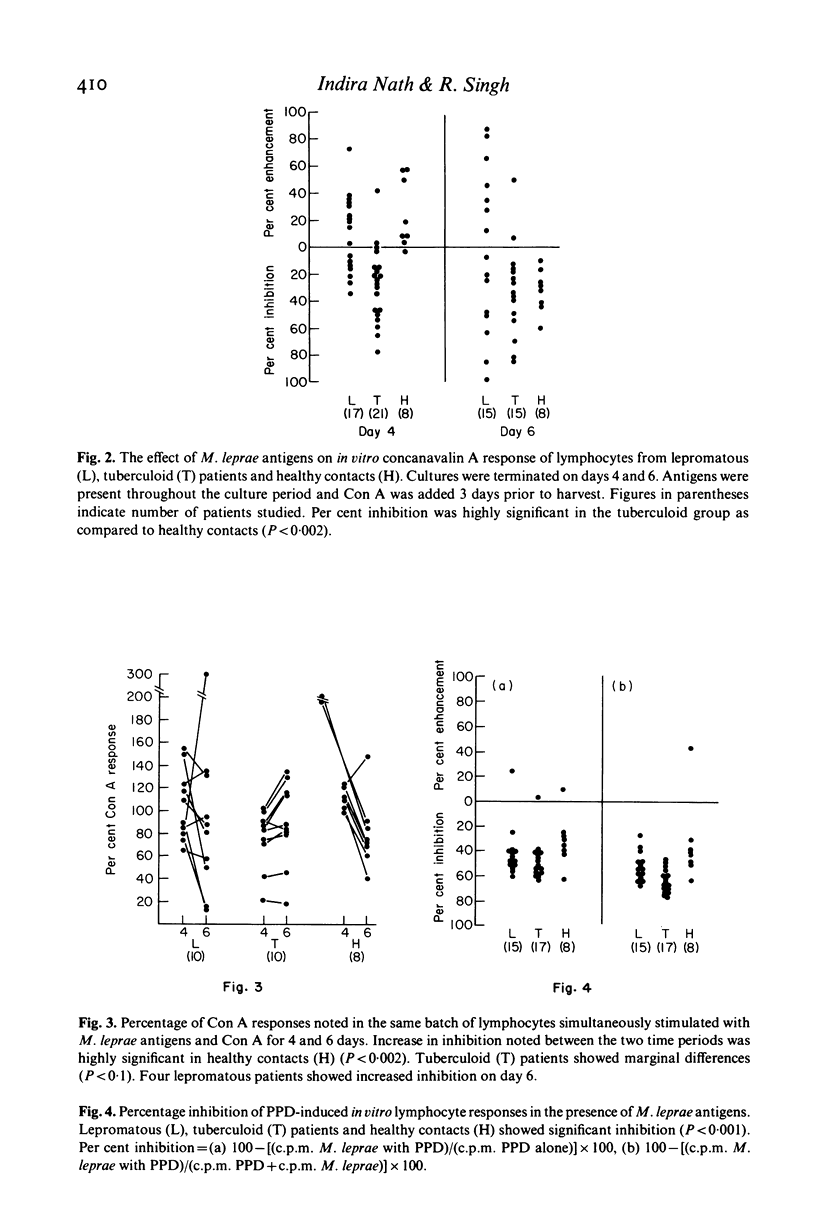
Peripheral blood lymphocytes from sixty leprosy patients and eight healthy contacts known to be responsive to M. leprae, were stimulated in vitro with concanavalin A (Con A) or PPD alone or in combination with autoclaved, whole M. leprae. Time kinetics and the percentage of inhibition induced by M. leprae differed in the two disease groups and contacts. Antigen-generated suppression of Con A-stimulated lymphocyte transformation was observed on day 4 in seventeen of twenty-one (80%) tuberculoid patients and six of seventeen (35.3%) untreated lepromatous patients. Healthy contacts and 53% lepromatous individuals showed enhanced Con A responses in the presence of antigen. On prolongation of antigen presence to 6 days, a marginal effect was noted in the tuberculoid group. In contrast, all healthy individuals and some lepromatous patients showed increased inhibition of Con A responses. M. leprae antigens showed uniform inhibition of PPD-induced 3H-thymidine incorporation in leprosy patients and healthy contacts.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Alexander J. Effect of cyclophosphamide treatment on the course of Mycobacterium lepraemurium infection and development of delayed-type hypersensitivity reactions in C57B1 and BALB/c mice. Clin Exp Immunol. 1978 Oct;34(1):52–58. [PMC free article] [PubMed] [Google Scholar]
- Barnetson R. S., Bjune G., Pearson J. M., Kronvall G. Antigenic heterogeneity in patients with reactions in borderline leprosy. Br Med J. 1975 Nov 22;4(5994):435–437. doi: 10.1136/bmj.4.5994.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjune G., Barnetson R. S., Ridley D. S., Kronvall G. Lymphocyte transformation test in leprosy; correlation of the response with inflammation of lesions. Clin Exp Immunol. 1976 Jul;25(1):85–94. [PMC free article] [PubMed] [Google Scholar]
- Bjune G. In vitro lymphocyte stimulation in leprosy; simultaneous stimulation with Mycobacterium leprae antigens and phytohaemagglutinin. Clin Exp Immunol. 1979 Jun;36(3):479–487. [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Godal T. Immunological aspects of leprosy--present status. Prog Allergy. 1978;25:211–242. [PubMed] [Google Scholar]
- Hansson M., Bakacs K. K., Kiessling R., Klein G. Intra- and interspecies reactivity of human and mouse natural killer (NK) cells. J Immunol. 1978 Jul;121(1):6–12. [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjune G., Kronvall G., Axelsen N. H. Mycobacterium leprae specific antibodies detected by radioimmunoassay. Scand J Immunol. 1978;7(2):111–120. doi: 10.1111/j.1365-3083.1978.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Hirschberg H. The role of macrophages in the lymphoproliferative response to Mycobacterium leprae in vitro. Clin Exp Immunol. 1978 Oct;34(1):46–51. [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Pelley R. P., Warren K. S. Immunoregulation in chronic infectious disease: schistosomiasis as a model. J Invest Dermatol. 1978 Jul;71(1):49–55. doi: 10.1111/1523-1747.ep12543940. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Nath I. Reduction of a subset of T cells bearing Fc receptors for IgG in lepromatous leprosy. Int Arch Allergy Appl Immunol. 1980;62(1):81–85. doi: 10.1159/000232490. [DOI] [PubMed] [Google Scholar]
- Stanford J. L., Rook G. A., Convit J., Godal T., Kronvall G., Rees R. J., Walsh G. P. Preliminary taxonomic studies on the leprosy bacillus. Br J Exp Pathol. 1975 Dec;56(6):579–585. [PMC free article] [PubMed] [Google Scholar]
- Stoner G. L. Importance of the neural predilection of Mycobacterium leprae in leprosy. Lancet. 1979 Nov 10;2(8150):994–996. doi: 10.1016/s0140-6736(79)92564-9. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]


