Abstract
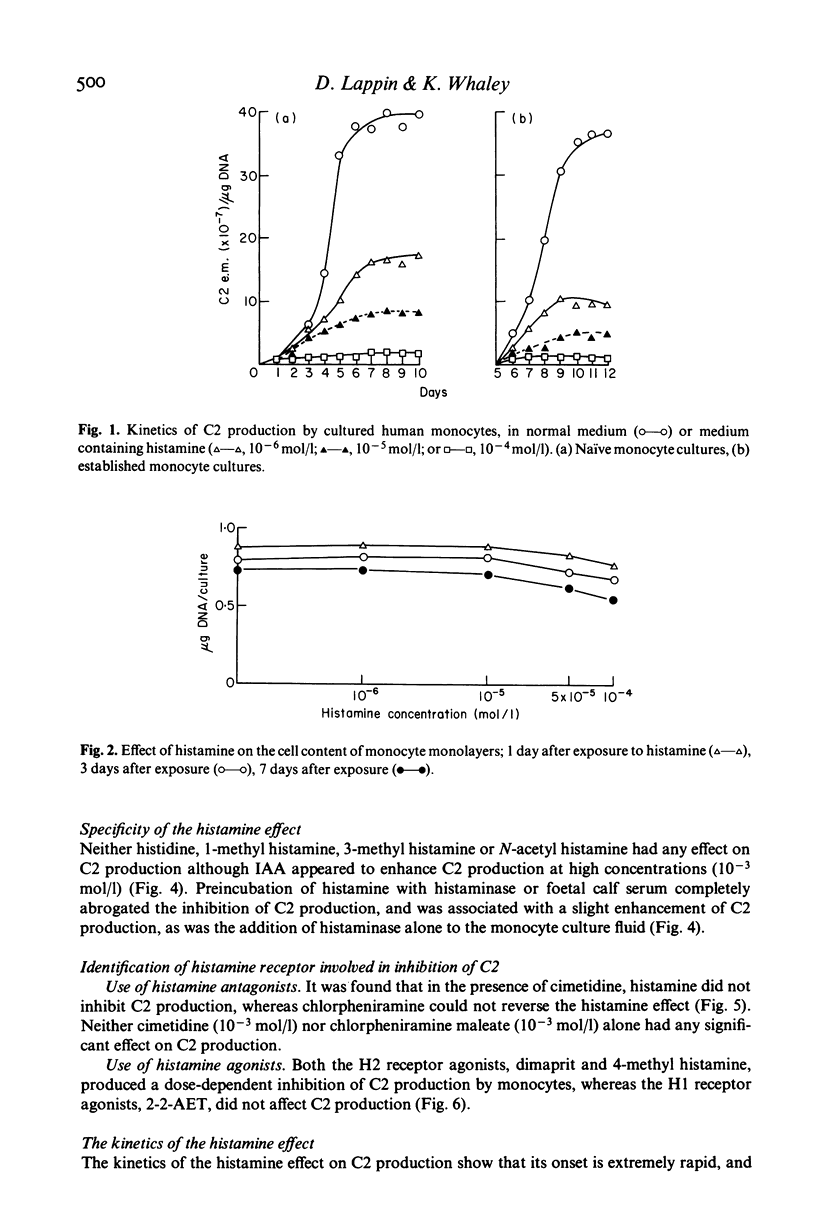
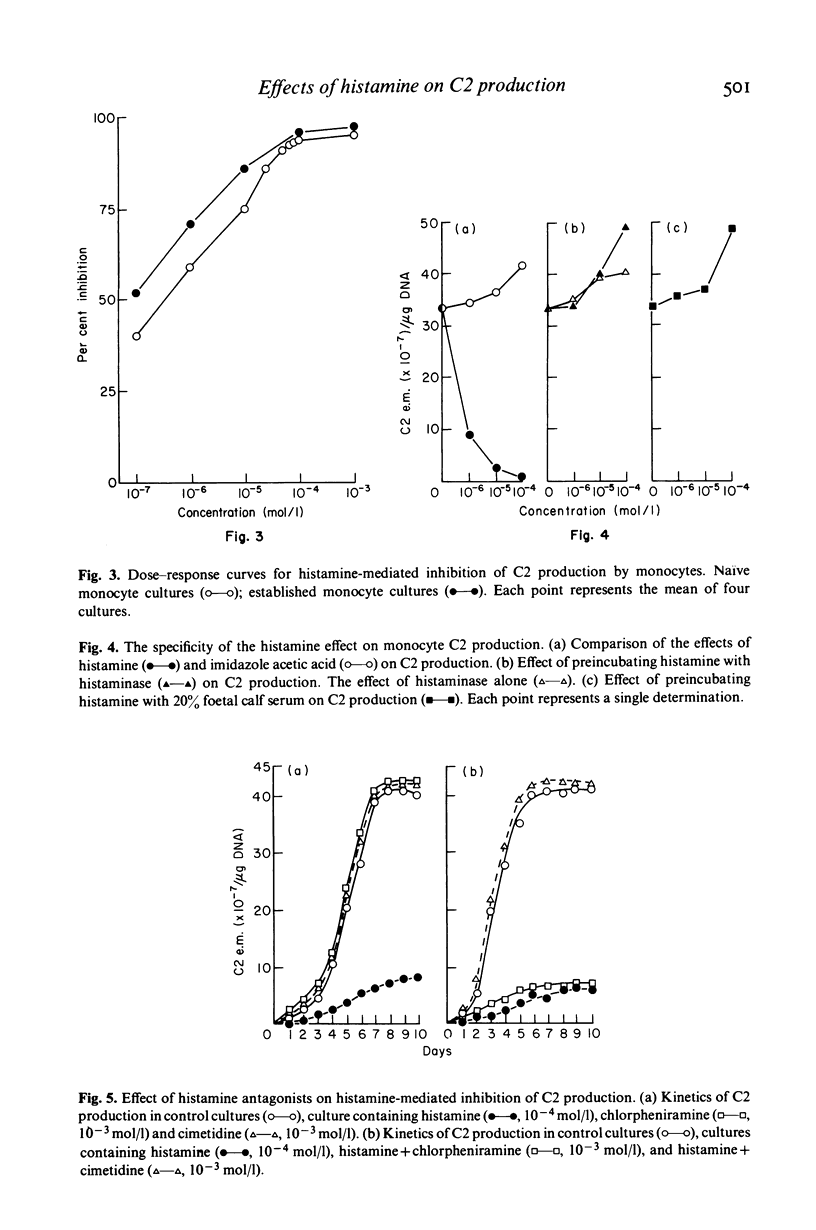
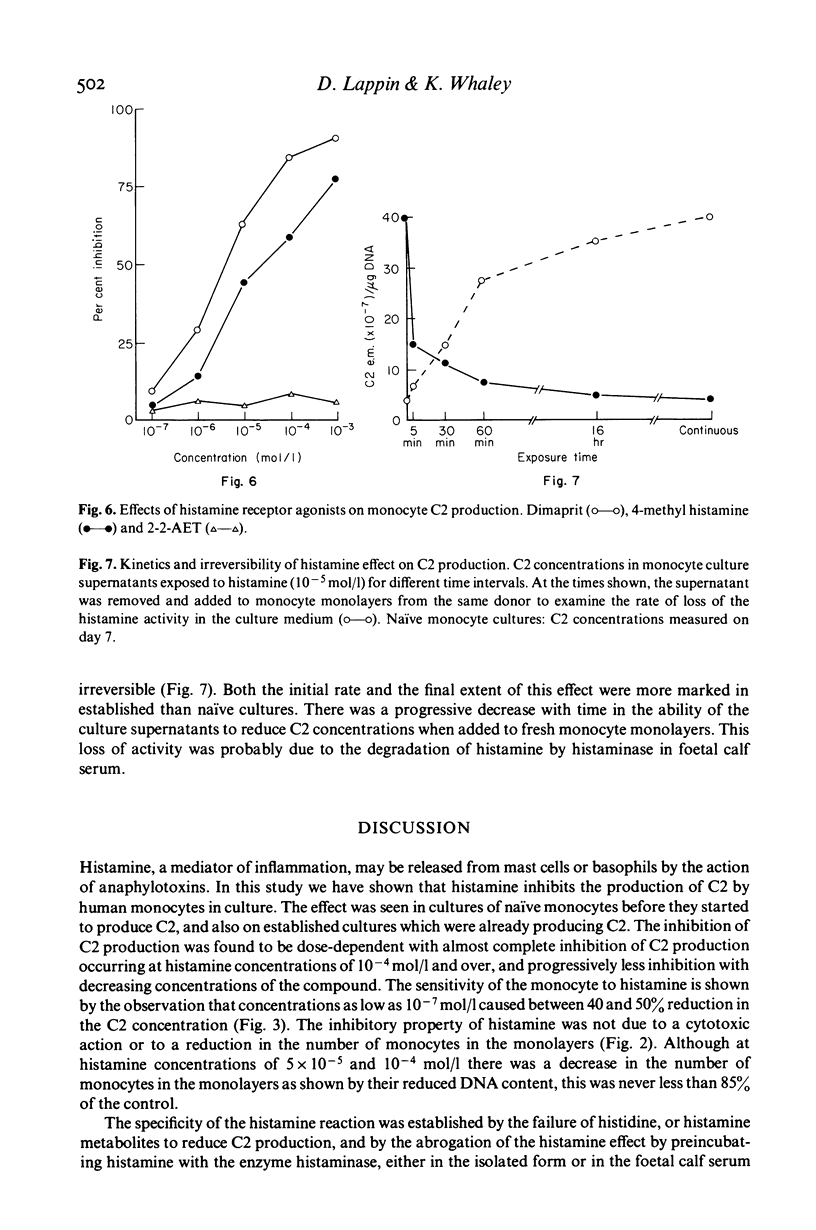
Histamine produced dose-dependent inhibition of the production of the second complement component (C2) by monocytes in tissue culture. The effect was not associated with either cell death, as ascertained by trypan blue exclusion, or loss of cells from the monolayer, as determined by measuring their DNA content. The specificity of the response was shown by the failure of histidine or histamine metabolites to inhibit C2 production. Preincubation of histamine with histaminase also abrogated the histamine effect. The kinetics of the effect were extremely rapid and irreversible, most of the reduction being achieved during a 5-min exposure to histamine. The H2 receptor antagonist cimetidine was able to prevent the histamine response, whereas chlorpheniramine, the H1 receptor antagonist, had no effect. Dimaprit and 4-methyl histamine, H2 receptor agonists, simulated the effect of histamine whereas the H1 receptor agonist 2-(2-aminoethylthiazole) was ineffective, confirming that the effect of histamine on C2 production by monocytes is mediated by the H2 receptors. Thus histamine, released from basophils or mast cells by the C3 and C5 cleavage products C3a and C5a respectively, may exert a negative feedback on further C3 and C5 cleavage by limiting the formation of the C3 (C42) and C5 (C423b) convertases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballet J. J., Merler E. The separation and reactivity in vitro of a subpopulation of human lymphocytes which bind histamine. Correlation of histamine reactivity with cellular maturation. Cell Immunol. 1976 Jun 15;24(2):250–269. doi: 10.1016/0008-8749(76)90210-0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Melmon K. L., Lichtenstein L. M. Histamine augments leukocyte adenosine 3',5'-monophosphate and blocks antigenic histamine release. Science. 1971 Aug 20;173(3998):743–745. doi: 10.1126/science.173.3998.743. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eosinophils and mediators of anaphylaxis. Histamine and imidazole acetic acid as chemotactic agents for human eosinophil leucocytes. Immunology. 1976 Nov;31(5):797–802. [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Lichtenstein L. M., Gillespie E. Inhibition of histamine release by histamine controlled by H2 receptor. Nature. 1973 Aug 3;244(5414):287–288. doi: 10.1038/244287a0. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein J., Sela M. Receptors for histamine can be detected on the surface of selected leukocytes. Science. 1972 Aug 25;177(4050):707–709. doi: 10.1126/science.177.4050.707. [DOI] [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Henney C. S. Increase in histamine receptors on thymus-derived effector lymphocytes during the primary immune response to alloantigens. Nature. 1973 Aug 3;244(5414):284–287. doi: 10.1038/244284a0. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Greineder D. K., Melmon K. L. Histamine-induced suppressor factor (HSF): further studies on the nature of the stimulus and the cell which produces it. Cell Immunol. 1979 May;44(2):404–415. doi: 10.1016/0008-8749(79)90015-7. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Greineder D., Littman B. H., Melmon K. L. Modulation of cellular immune function in vitro by histamine receptor-bearing lymphocytes: mechanism of action. Cell Immunol. 1978 Apr;37(1):162–173. doi: 10.1016/0008-8749(78)90184-3. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E. Modulation of cellular-immune responses in vivo and in vitro by histamine receptor-bearing lymphocytes. J Clin Invest. 1976 Apr;57(4):1051–1058. doi: 10.1172/JCI108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. A stoichiometric assay for the fourth component of complement in whole human serum using EAC'la-gp and functionally pure human second component. J Immunol. 1967 Dec;99(6):1162–1172. [PubMed] [Google Scholar]
- Saxon A., Morledge V. D., Bonavida B. Histamine-receptor leucocytes (HRL). Organ and lymphoid subpopulation distribution in man. Clin Exp Immunol. 1977 Jun;28(3):394–399. [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Melmon K. L., Weinstein Y., Sela M. Regulation of antibody response by cells expressing histamine receptors. J Exp Med. 1972 Nov 1;136(5):1302–1307. doi: 10.1084/jem.136.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Melmon K. L., Bourne H. R., Sela M. Specific leukocyte receptors for small endogenous hormones. Detection by cell binding to insolubilized hormone preparations. J Clin Invest. 1973 Jun;52(6):1349–1361. doi: 10.1172/JCI107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J Exp Med. 1980 Mar 1;151(3):501–516. doi: 10.1084/jem.151.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]


