Abstract
Spleen cells from DBA/2 mice immunized with high numbers of sheep red blood cells specifically suppress the primary anti-SRBC antibody response of syngeneic recipients specifically suppress the primary anti-SRBC antibody response of syngeneic recipients after in vivo transfer. Such suppressive activity of the immune spleen cells is mediated by null cells, or by T cells resistant to the cytotoxic activity of anti-Thy 1.2 antiserum plus complement. The primary anti-SRBC antibody response is much higher in NZB mice than in DBA/2 mice, and the suppressive activity of syngeneic immune spleen cells is much lower in NZB than in DBA/2 recipients. Immune spleen cells from DBA/2 donors do not provide more effective suppression than NZB spleen cells in NZB recipients. Conversely, immune spleen cells from NZB donors strongly suppress the anti-SRBC primary response of DBA/2 recipients to the same extent as DBA/2 immune spleen cells. Finally, NZB mice generate specific suppressor cells but their primary antibody response is not sensitive to this suppressor activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A. Differences in Cyclic AMP Changes after Stimulation by Prostaglandins and Isoproterenol in Lymphocyte Subpopulations. J Clin Invest. 1975 May;55(5):1074–1081. doi: 10.1172/JCI108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Loblay R., Johnson P., Gamble J., Chia E., Pritchard-Briscoe H., Callard R., McKenzie I. F. T cell-dependent suppression of antibody production. I. Characteristics of suppressor T cells following tolerance induction. Eur J Immunol. 1978 May;8(5):360–370. doi: 10.1002/eji.1830080513. [DOI] [PubMed] [Google Scholar]
- Cantor H., McVay-Boudreau L., Hugenberger J., Naidorf K., Shen F. W., Gershon R. K. Immunoregulatory circuits among T-cell sets. II. Physiologic role of feedback inhibition in vivo: absence in NZB mice. J Exp Med. 1978 Apr 1;147(4):1116–1125. doi: 10.1084/jem.147.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
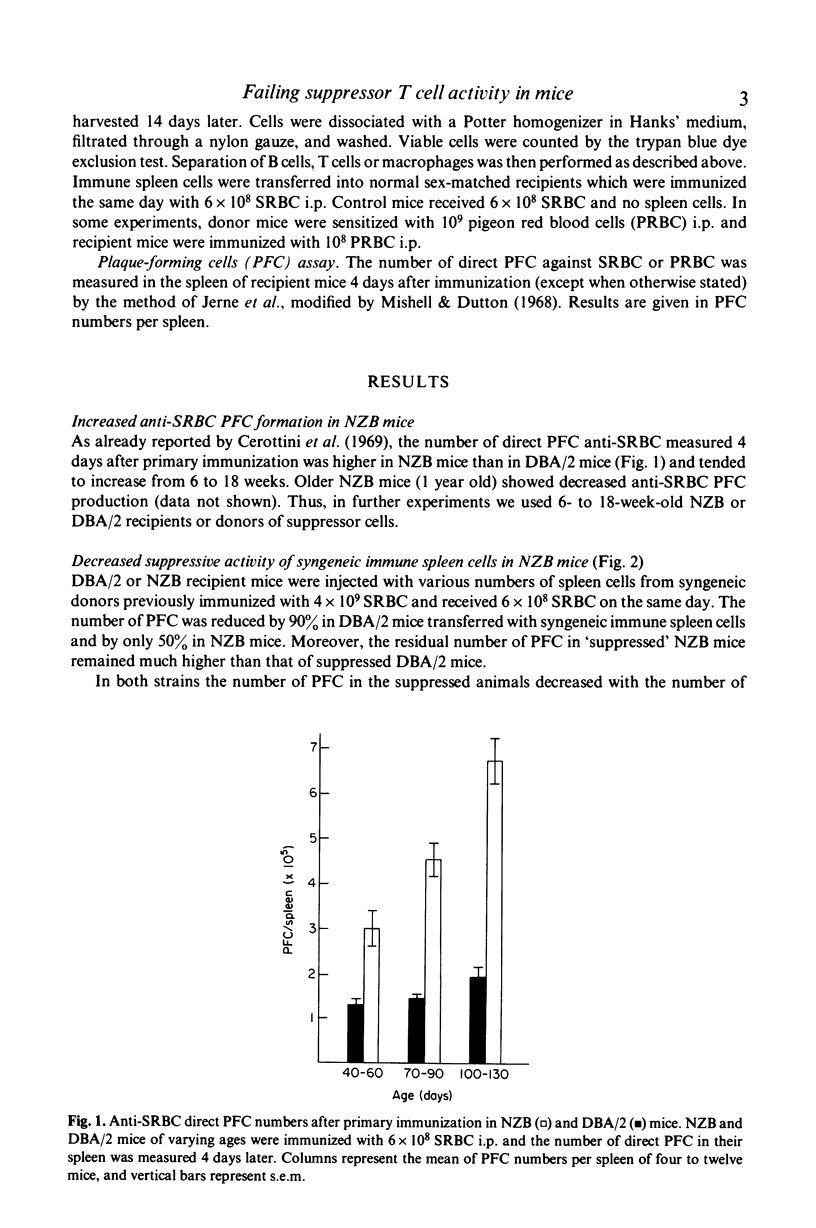
- Cerottini J. C., Lambert P. H., Dixon F. J. Comparison of the immune responsiveness of NZB and NZB X NZW F1 hybrid mice with that of other strains of mice. J Exp Med. 1969 Nov 1;130(5):1093–1105. doi: 10.1084/jem.130.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Ziff M., Vitetta E. S. Characterization of a B cell defect in the NZB mouse manifested by an increased ratio of surface IgM to IgD. J Immunol. 1978 Sep;121(3):973–977. [PubMed] [Google Scholar]
- Cornain S., Carnaud C., Silverman D., Klein E., Rajewsky M. F. Spleen-cell reactivity against transplanted neurogenic rat tumors induced by ethylnitrosourea: uncovering of tumor specificity after removal of complement-receptor-bearing lymphocytes. Int J Cancer. 1975 Aug 15;16(2):301–311. doi: 10.1002/ijc.2910160213. [DOI] [PubMed] [Google Scholar]
- Dauphinée M. J., Talal N. Failure of NZB spleen to respond to prethymic bone marrow suppressor cells. J Immunol. 1979 Mar;122(3):936–941. [PubMed] [Google Scholar]
- Eardley D. D., Gershon R. K. Induction of specific suppressor T cells in vitro. J Immunol. 1976 Jul;117(1):313–318. [PubMed] [Google Scholar]
- Feldmann M., Beverley P. C., Woody J., McKenzie I. F. T-T interactions in the induction of suppressor and helper T cells: analysis of membrane phenotype of precursor and amplifier cells. J Exp Med. 1977 Apr 1;145(4):793–801. doi: 10.1084/jem.145.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Ikeda R. M., Kruse W. L., Wilson F., Shifrine M., Spangler W. Age-dependent loss in New Zealand mice of morphological and functional characteristics of thymic epithelial cells. J Immunol. 1978 Mar;120(3):971–979. [PubMed] [Google Scholar]
- Gershwin M. E., Steinberg A. D. Suppression of autoimmune hemolytic anemia in New Zealand (NZB) mice by syngeneic young thymocytes. Clin Immunol Immunopathol. 1975 May;4(1):38–45. doi: 10.1016/0090-1229(75)90037-9. [DOI] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Lobo P. I., Westervelt F. B., Horwitz D. A. Identification of two populations of immunoglobulin-bearing lymphocytes in man. J Immunol. 1975 Jan;114(1 Pt 1):116–119. [PubMed] [Google Scholar]
- Moutsopoulos H. M., Boehm-Truitt M., Kassan S. S., Chused T. M. Demonstration of activation of B lymphocytes in New Zealand black mice at birth by an immunoradiometric assay for murine IgM. J Immunol. 1977 Nov;119(5):1639–1644. [PubMed] [Google Scholar]
- Playfair J. H. Strain differences in the immune response of mice. I. The neonatal response to sheep red cells. Immunology. 1968 Jul;15(1):35–50. [PMC free article] [PubMed] [Google Scholar]
- Rytel M. W., Lytle R. I. Immunoconglutinin response in patients with acute viral respiratory disease. Clin Exp Immunol. 1973 Jun;14(2):227–235. [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1412–1415. doi: 10.1073/pnas.68.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Wang A. L., Rutishauser U., Edelman G. M. Characterization of splenic lymphoid cells in fetal and newborn mice. J Exp Med. 1973 Sep 1;138(3):557–573. doi: 10.1084/jem.138.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talal N., Steinberg A. D. The pathogenesis of autoimmunity in New Zealand black mice. Curr Top Microbiol Immunol. 1974;64(0):79–103. doi: 10.1007/978-3-642-65848-8_3. [DOI] [PubMed] [Google Scholar]
- Whisler R. L., Stobo J. D. Heterogeneity of murine regulatory T cells. I. Subpopulations of amplifier and suppressor T cells. J Exp Med. 1976 Aug 1;144(2):398–413. doi: 10.1084/jem.144.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- Yuan D., Vitetta E. S., Kettman J. R. Cell surface immunoglobulin. XX. Antibody responsiveness of subpopulations of B lymphocytes bearing different isotypes. J Exp Med. 1977 Jun 1;145(6):1421–1435. doi: 10.1084/jem.145.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]


