Abstract
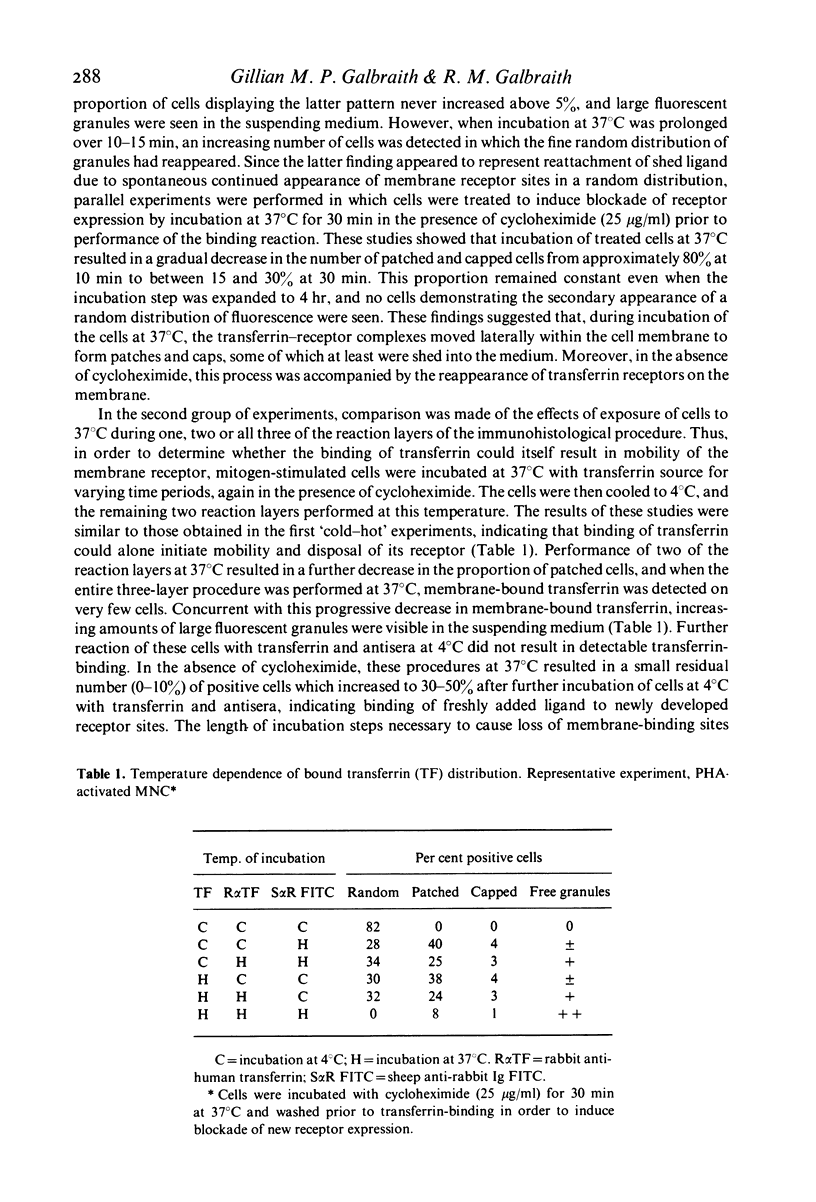
The transferrin receptors which appear on mitogen-activated human peripheral blood lymphocytes were found by the use of immunofluorescence techniques to display temperature-dependent patching and capping reactions upon binding of transferrin. Lateral mobility of ligand-occupied membrane sites was accompanied by both shedding and endocytosis of receptor-transferrin complexes. In the presence of sodium azide or the microfilament inhibitor cytochalasin B, cap formation and shedding were markedly inhibited. In contrast, endocytosis of patched receptor-ligand complexes was inhibited by azide and microtubule inhibitors, including colchicine, vinblastine and vincristine. Co-capping experiments performed to elucidate further the alterations in membrane configuration involved in these reactions failed to reveal any topographical relationship between transferrin receptors and lectin-binding sites in these cells. These studied indicate that temperature-dependent mobility of transferrin receptors upon mitogen-activated peripheral blood lymphocytes is dependent upon the integrity of the cytoskeletal system and metabolic function of the cell.
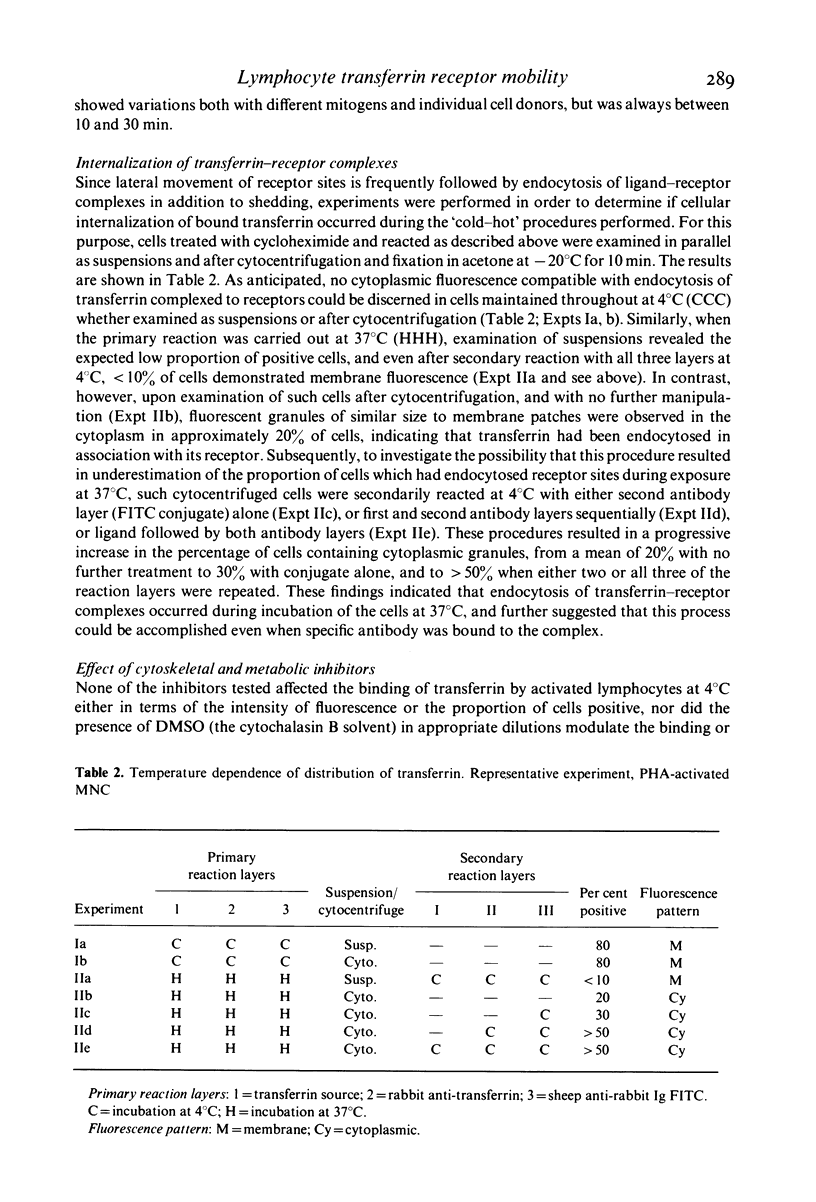
Full text
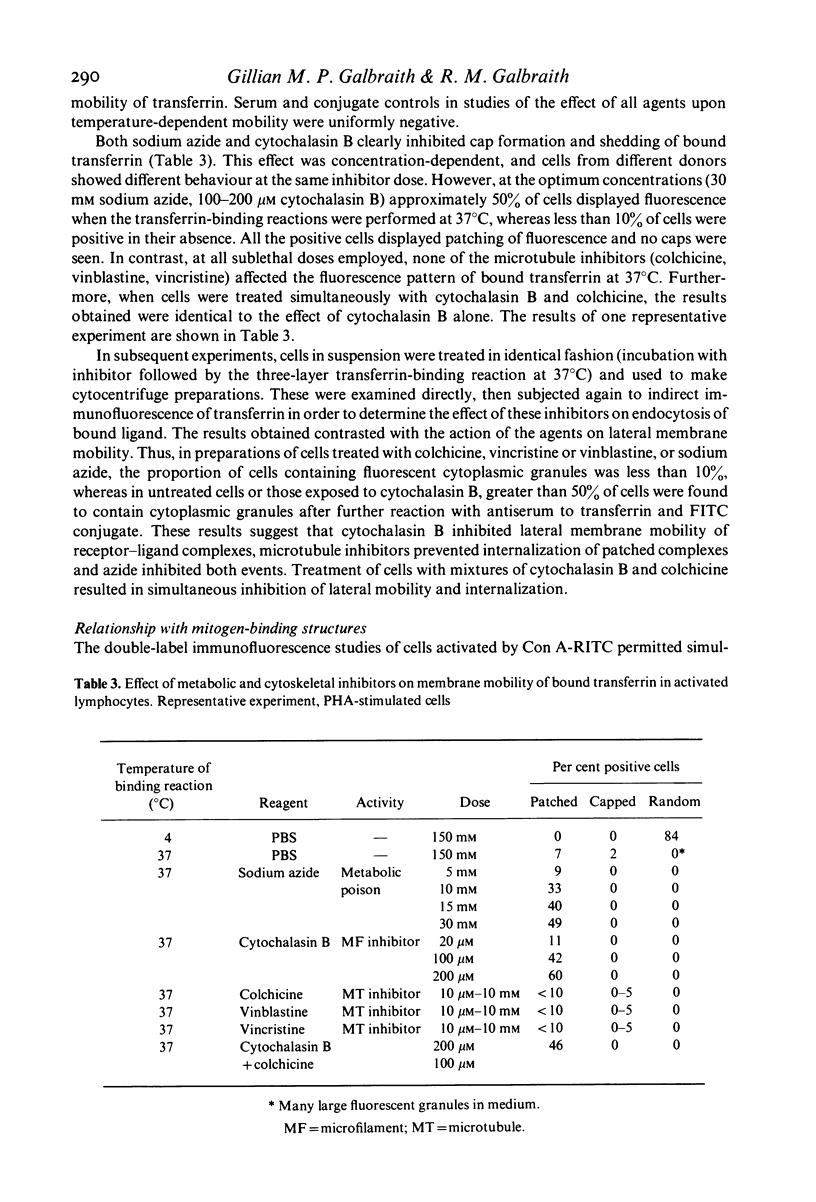
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Petris S. Inhibition and reversal of capping by cytochalasin B, vinblastine and colchicine. Nature. 1974 Jul 5;250(461):54–56. doi: 10.1038/250054a0. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Faulk W. P. Immunological studies of transferrin and transferrin receptors of human placental trophoblast. Placenta. 1980 Jan-Mar;1(1):33–46. doi: 10.1016/s0143-4004(80)80014-2. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Faulk W. P. Transferrin binding by human lymphoblastoid cell lines and other transformed cells. Cell Immunol. 1980 Jan;49(1):215–222. doi: 10.1016/0008-8749(80)90072-6. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Temple A., Faulk W. P. Demonstration of transferrin receptors on human placental trophoblast. Blood. 1980 Feb;55(2):240–242. [PubMed] [Google Scholar]
- Hemmaplardh D., Kailis S. G., Morgan E. H. The effects of inhibitors of microtubule and microfilament function on transferrin and iron uptake by rabbit reticulocytes and bone marrow. Br J Haematol. 1974 Sep;28(1):53–65. doi: 10.1111/j.1365-2141.1974.tb06639.x. [DOI] [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. The role of endocytosis in transferrin uptake by reticulocytes and bone marrow cells. Br J Haematol. 1977 May;36(1):85–96. doi: 10.1111/j.1365-2141.1977.tb05758.x. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Transferrin receptors on human B and T lymphoblastoid cell lines. Biochim Biophys Acta. 1979 Apr 3;583(4):483–490. doi: 10.1016/0304-4165(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Poste G. The cancer cell: dynamic aspects and modifications in cell-surface organization (first of two parts). N Engl J Med. 1976 Jul 22;295(4):197–203. doi: 10.1056/NEJM197607222950405. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Phillips J. L. Specific binding of zinc transferrin to human lymphocytes. Biochem Biophys Res Commun. 1976 Sep 20;72(2):634–639. doi: 10.1016/s0006-291x(76)80087-3. [DOI] [PubMed] [Google Scholar]
- Raff M. C., De Petris S. Movement of lymphocyte surface antigens and receptors: the fluid nature of the lymphocyte plasma membrane and its immunological significance. Fed Proc. 1973 Jan;32(1):48–54. [PubMed] [Google Scholar]
- Sundqvist K. G., Ehrnst A. Cytoskeletal control of surface membrane mobility. Nature. 1976 Nov 18;264(5583):226–231. doi: 10.1038/264226a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J. Redistribution and fate of Ig complexes on surface of B lymphocytes: functional implications and mechanisms. Transplant Rev. 1973;14:184–210. doi: 10.1111/j.1600-065x.1973.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petris S. Concanavalin A receptors, immunoglobulins, and theta antigen of the lymphocyte surface. Interactions with concanavalin A and with Cytoplasmic structures. J Cell Biol. 1975 Apr;65(1):123–146. doi: 10.1083/jcb.65.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]


