Abstract
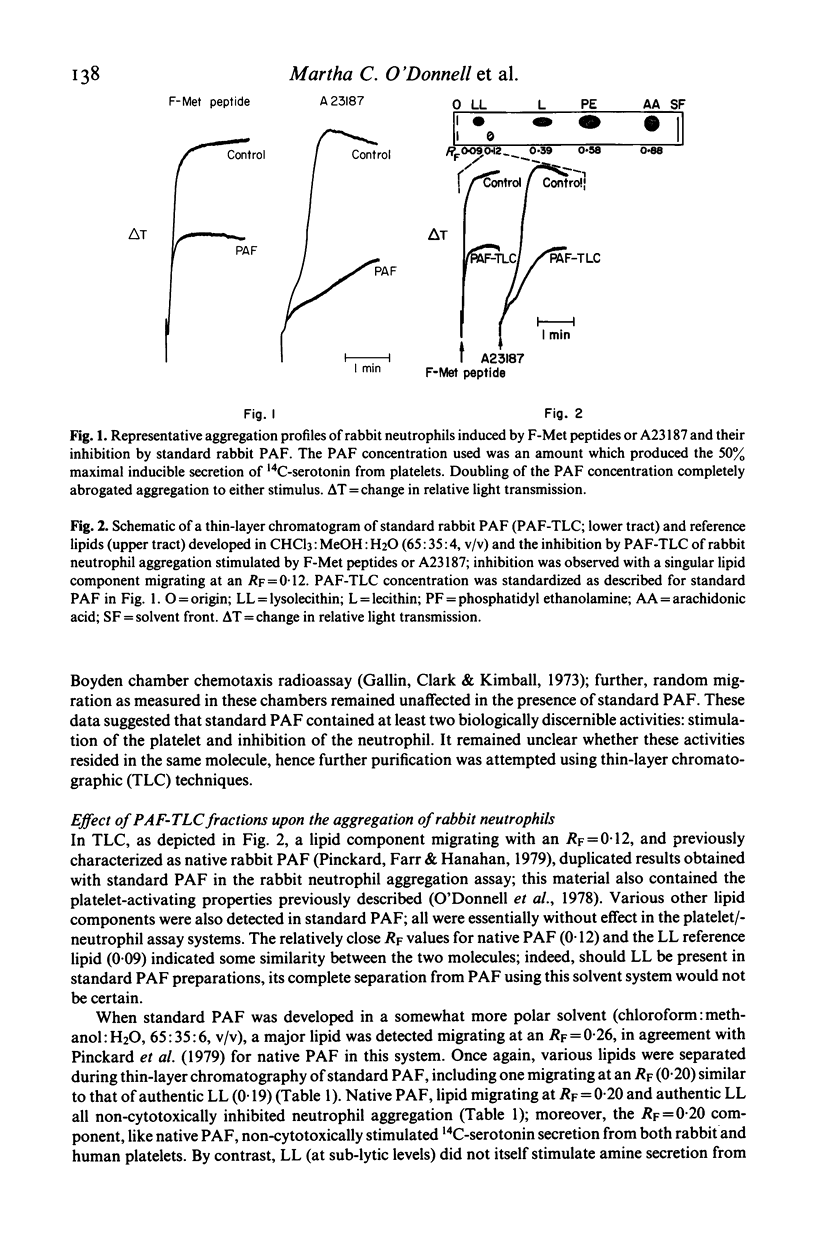
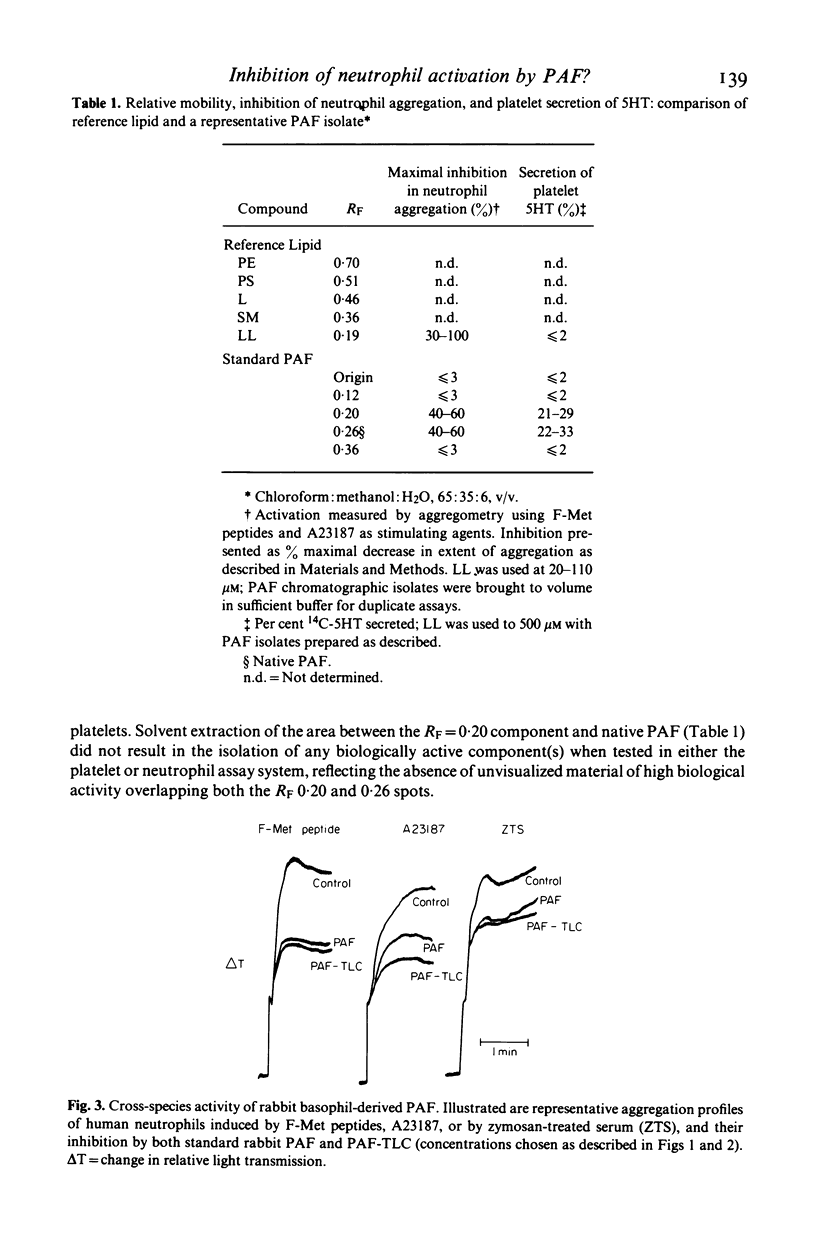
Preparations of platelet activating factor (PAF) derived by methanolic extraction of supernatants from antigen-challenged rabbit basophils proved capable of activating platelets while concurrently inhibiting neutrophil aggregation/secretion stimulated by biologically active F-Met peptides, ionophore A23187, or zymosan-treated serum. This inhibition was non-cytotoxic and species-non-specific. When these PAF preparations were analysed using thin-layer chromatography, multiple lipids were detected. Both platelet-stimulating as well as neutrophil-inhibitory activity was present in a lipid component migrating at an RF consistent with native PAF; however, these biological activities were not limited only to PAF and, indeed, could also be detected in lipid with solubility characteristics more closely related to a lysophosphatide than to native PAF. These data are compatible with the belief that native PAF may belong to a family of biologically active lipids differing somewhat in physico-chemical properties. Moreover, these data illustrate that PAF and/or PAF-like molecules may also demonstrate a biological activity distinct from their effects upon the platelet.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Le Couedic J. P., Kamoun P. Letter: Aggregation of human platelets by platelet-activating factor. Lancet. 1975 Feb 8;1(7902):344–345. doi: 10.1016/s0140-6736(75)91264-7. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Le Couedic J. P., Polonsky J., Tence M. Structural analysis of purified platelet-activating factor by lipases. Nature. 1977 Sep 8;269(5624):170–171. doi: 10.1038/269170a0. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Tencé M., Varenne P., Bidault J., Boullet C., Polonsky J. Semi-synthèse et structure proposée du facteur activant les plaquettes (P.A.F.): PAF-acether, un alkyl ether analogue de la lysophosphatidylcholine. C R Seances Acad Sci D. 1979 Nov 26;289(14):1037–1040. [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Mencia-Huerta J. M., Benveniste J. Release of platelet-activating factor and histamine. I. Effect of immune complexes, complement and neutrophils on human and rabbit mastocytes and basophils. Immunology. 1977 Oct;33(4):523–534. [PMC free article] [PubMed] [Google Scholar]
- Cazenave J. P., Benveniste J., Mustard J. F. Aggregation of rabbit platelets by platelet-activating factor is independent of the release reaction and the arachidonate pathway and inhibited by membrane-active drugs. Lab Invest. 1979 Sep;41(3):275–285. [PubMed] [Google Scholar]
- Chignard M., Le Couedic J. P., Tence M., Vargaftig B. B., Benveniste J. The role of platelet-activating factor in platelet aggregation. Nature. 1979 Jun 28;279(5716):799–800. doi: 10.1038/279799a0. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., White J. G., Jacob H. S. Potentiation of complement (C5a)-induced granulocyte aggregation by cytochalasin B. J Lab Clin Med. 1978 Mar;91(3):490–499. [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Fiedel B. A. Effects of lysolecithin on aggregation of human platelets induced by arachidonic acid and A23187 role of prostaglandin intermediary metabolism. Life Sci. 1978 Feb;22(6):531–534. doi: 10.1016/0024-3205(78)90435-6. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Henson P. M. Activation and desensitization of platelets by platelet-activating factor (PAF) derived from IgE-sensitized basophils. I. Characteristics of the secretory response. J Exp Med. 1976 Apr 1;143(4):937–952. doi: 10.1084/jem.143.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Activation of rabbit platelets by platelet-activating factor derived from IgE-sensitized basophils. Characteristics of the aggregation and its dissociation from secretion. J Clin Invest. 1977 Aug;60(2):481–490. doi: 10.1172/JCI108799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Acute immune complex disease in rabbits. The role of complement and of a leukocyte-dependent release of vasoactive amines from platelets. J Exp Med. 1971 Mar 1;133(3):554–571. doi: 10.1084/jem.133.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Release of vasoactive amines from rabbit platelets induced by sensitized mononuclear leukocytes and antigen. J Exp Med. 1970 Feb;131(2):287–306. doi: 10.1084/jem.131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Craddock P. R., Hammerschmidt D. E., Moldow C. F. Complement-induced granulocyte aggregation: an unsuspected mechanism of disease. N Engl J Med. 1980 Apr 3;302(14):789–794. doi: 10.1056/NEJM198004033021407. [DOI] [PubMed] [Google Scholar]
- Joist J. H., Dolezel G., Cucuianu M. P., Nishizawa E. E., Mustard J. F. Inhibition and potentiation of platelet function by lysolecithin. Blood. 1977 Jan;49(1):101–112. [PubMed] [Google Scholar]
- Kravis T. C., Henson P. M. Accumulation of platelets at sites of antigen-antibody-mediated injury: a possible role for IgE antibody and mast cells. J Immunol. 1977 May;118(5):1569–1573. [PubMed] [Google Scholar]
- Kravis T. C., Henson P. M. IgE-induced release of a platelet-activating factor from rabbit lung. J Immunol. 1975 Dec;115(6):1677–1681. [PubMed] [Google Scholar]
- Lian E. C., Harkness D. R., Byrnes J. J., Wallach H., Nunez R. Presence of a platelet aggregating factor in the plasma of patients with thrombotic thrombocytopenic purpura (TTP) and its inhibition by normal plasma. Blood. 1979 Feb;53(2):333–338. [PubMed] [Google Scholar]
- Lotner G. Z., Lynch J. M., Betz S. J., Henson P. M. Human neutrophil-derived platelet activating factor. J Immunol. 1980 Feb;124(2):676–684. [PubMed] [Google Scholar]
- Lynch J. M., Lotner G. Z., Betz S. J., Henson P. M. The release of a platelet-activating factor by stimulated rabbit neutrophils. J Immunol. 1979 Sep;123(3):1219–1226. [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. C., Henson P. M., Fiedel B. A. Activation of human platelets by platelet activating factor (PAF) derived from sensitized rabbit basophils. Immunology. 1978 Dec;35(6):953–958. [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Showell H. J., Ward P. A. Influence of inhibitors of cellular function on chemotactic factor-induced neutrophil aggregation. J Immunol. 1977 Nov;119(5):1751–1756. [PubMed] [Google Scholar]
- Pinckard R. N., Farr R. S., Hanahan D. J. Physicochemical and functional identity of rabbit platelet-activating factor (PAF) released in vivo during IgE anaphylaxis with PAF released in vitro from IgE sensitized basophils. J Immunol. 1979 Oct;123(4):1847–1857. [PubMed] [Google Scholar]
- Pinckard R. N., Halonen M., Palmer J. D., Butler C., Shaw J. O., Henson P. M. Intravascular aggregation and pulmonary sequestration of platelets during IgE-induced systemic anaphylaxis in the rabbit: abrogation of lethal anaphylactic shock by platelet depletion. J Immunol. 1977 Dec;119(6):2185–2193. [PubMed] [Google Scholar]
- Shaw J. O., Printz M. P., Hirabayashi K., Henson P. M. Role of prostaglandin synthesis in rabbit platelet activation induced by basophil-derived platelet-activating factor. J Immunol. 1978 Nov;121(5):1939–1945. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]


