Abstract
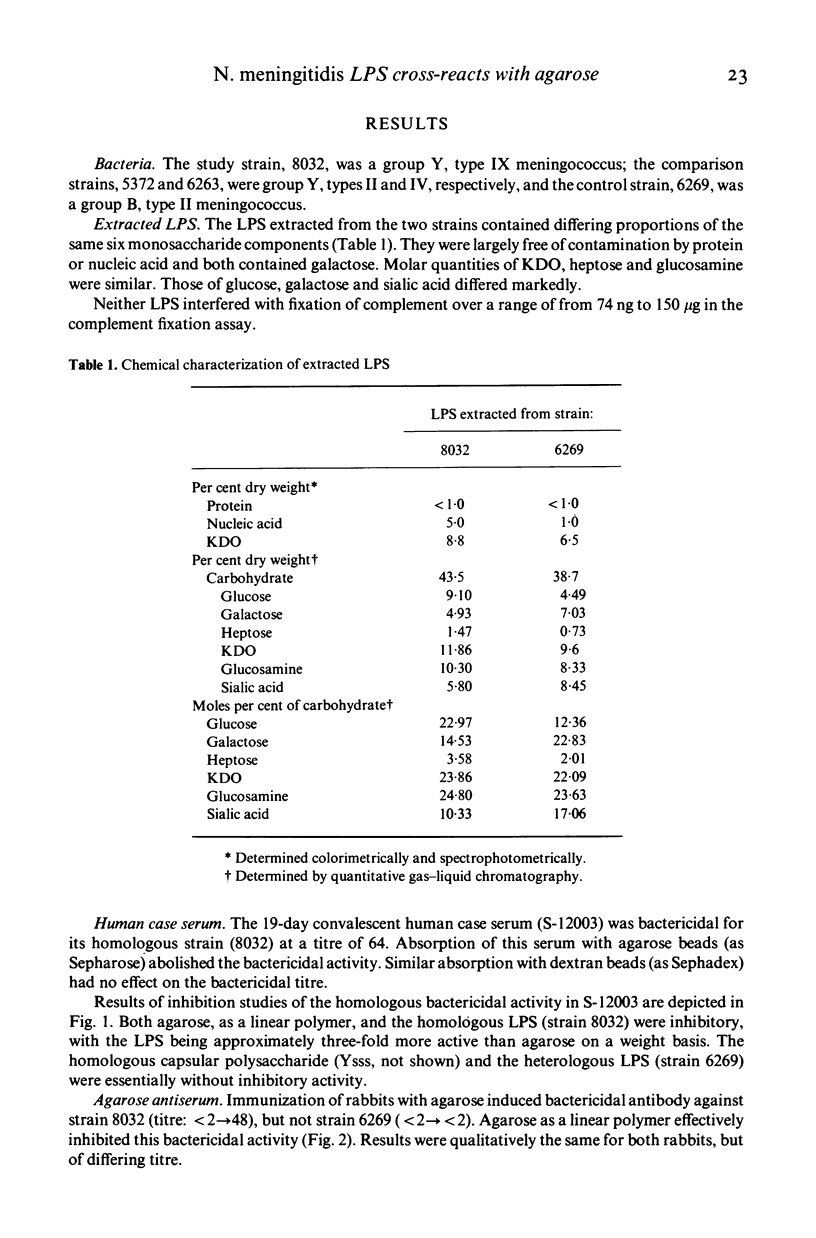
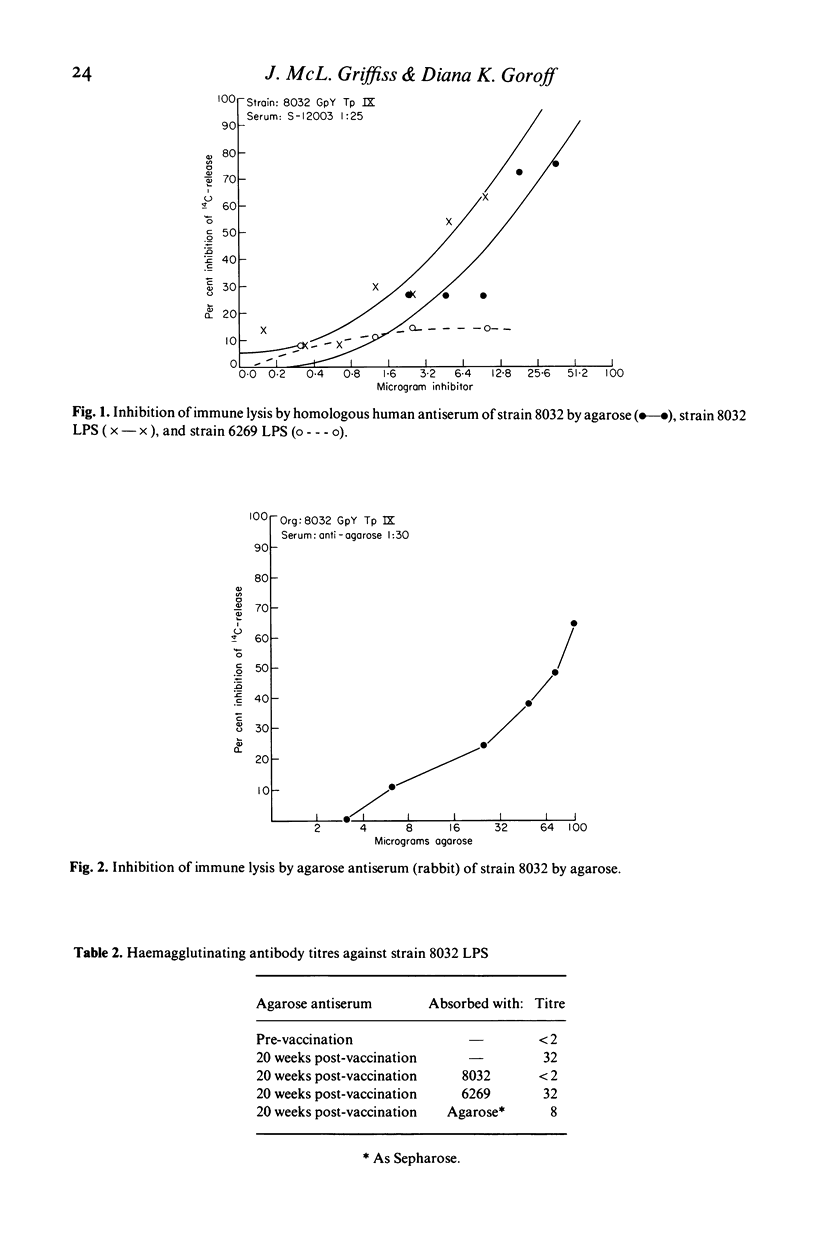
An immunological cross-reaction between agarose, a naturally occurring galactan, and an antigenic determinant which is a locus for human bactericidal antibody within the LPS of group Y strains of N. meningitidis was investigated. Bactericidal antibody in the convalescent serum of a child from whom a group Y, type IX strain was isolated could be absorbed by highly purified agarose in bead form (Sepharose), but not by a dextran gel (Sephadex). It was inhibited by agarose as a linear polymer and by the strain's LPS, but not by a heterologous LPS from a group B, type II strain of N. meningitidis, nor by the homologous capsular polysaccharide. Both LPS contained galactose; neither was anti-complementary. Agarose antiserum, raised in a rabbit, was bactericidal for the group Y strain, but not for the group B strain. Bactericidal activity in the agarose antiserum could be inhibited by agarose. Immunization with agarose induced haemagglutinating antibody against the group Y strain's LPS which could be absorbed by the group Y, but not the group B strain, and could be reduced by absorption with Sepharose. Absorption with Sepharose also removed the lytic activity against group Y strains of serotypes II and IV from a normal human serum without group Y capsular polysaccharide antibody. We conclude that the cross-reaction resides in the fine structure of the galactose constituent of the group Y strains' LPS.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Fulmer A., Scott W. E., Dea I. C., Moorhouse R., Rees D. A. The agarose double helix and its function in agarose gel structure. J Mol Biol. 1974 Dec 5;90(2):269–284. doi: 10.1016/0022-2836(74)90372-6. [DOI] [PubMed] [Google Scholar]
- Berman S., Altieri P. L., Groffinger A., Lowenthal J. P. Pilot-scale production of group a and group C meningococcal polysaccharide immunogens. Infect Immun. 1970 Nov;2(5):640–643. doi: 10.1128/iai.2.5.640-643.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram M. A., Griffiss J. M., Broud D. D. Response to antigenic determinants of Neisseria meningitidis lipopolysaccharide investigated with a new radioactive antigen-binding assay. J Immunol. 1976 Mar;116(3):842–846. [PubMed] [Google Scholar]
- Bhattacharjee A. K., Jennings H. J., Kenny C. P., Martin A., Smith I. C. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can J Biochem. 1976 Jan;54(1):1–8. doi: 10.1139/o76-001. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Jennings H. J., Kenny C. P., Martin A., Smith I. C. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975 Mar 10;250(5):1926–1932. [PubMed] [Google Scholar]
- Brandt B. L., Wyle F. A., Artenstein M. S. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972 Apr;108(4):913–920. [PubMed] [Google Scholar]
- Broud D. D., Griffiss J. M., Baker C. J. Heterogenity of serotypes of Neisseria meningitidis that cause endemic disease. J Infect Dis. 1979 Oct;140(4):465–470. doi: 10.1093/infdis/140.4.465. [DOI] [PubMed] [Google Scholar]
- Evans J. R., Artenstein M. S., Hunter D. H. Prevalence of meningococcal serogroups and description of three new groups. Am J Epidemiol. 1968 May;87(3):643–646. doi: 10.1093/oxfordjournals.aje.a120854. [DOI] [PubMed] [Google Scholar]
- Glode M. P., Robbins J. B., Liu T. Y., Gotschlich E. C., Orskov I., Orskov F. Cross-antigenicity and immunogenicity between capsular polysaccharides of group C Neisseria meningitidis and of Escherichia coli K92. J Infect Dis. 1977 Jan;135(1):94–104. doi: 10.1093/infdis/135.1.94. [DOI] [PubMed] [Google Scholar]
- Gold R., Goldschneider I., Lepow M. L., Draper T. F., Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978 Feb;137(2):112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- Gold R., Winklehake J. L., Mars R. S., Artenstein M. S. Identification of an epidemic strain of group C Neisseria meningitidis by bactericidal serotyping. J Infect Dis. 1971 Dec;124(6):593–597. doi: 10.1093/infdis/124.6.593. [DOI] [PubMed] [Google Scholar]
- Gold R., Wyle F. A. New Classification of Neisseria meningitidis by Means of Bactericidal Reactions. Infect Immun. 1970 May;1(5):479–484. doi: 10.1128/iai.1.5.479-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Bertram M. A. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J Infect Dis. 1977 Dec;136(6):733–739. doi: 10.1093/infdis/136.6.733. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Broud D. D., Silver C. A., Artenstein M. S. Immunoepidemiology of meningococcal disease in military recruits. I. A model for serogroup independency of epidemic potential as determined by serotyping. J Infect Dis. 1977 Aug;136(2):176–186. doi: 10.1093/infdis/136.2.176. [DOI] [PubMed] [Google Scholar]
- HJERTEN S. THE PREPARATION OF AGAROSE SPHERES FOR CHROMATOGRAPHY OF MOLECULES AND PARTICLES. Biochim Biophys Acta. 1964 Mar 30;79:393–398. [PubMed] [Google Scholar]
- HOOK W. A., MUSCHEL L. H. ANTICOMPLEMENTARY EFFECTS AND COMPLEMENT ACTIVITY OF HUMAN SERA. Proc Soc Exp Biol Med. 1964 Oct;117:292–297. doi: 10.3181/00379727-117-29561. [DOI] [PubMed] [Google Scholar]
- Jenkin C. R., Karnovsky M. L., Rowley D. Preparation of an artificial antigen and immunity to mouse typhoid. Immunology. 1967 Oct;13(4):361–372. [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Hawes G. B., Adams G. A., Kenny C. P. The chemical composition and serological reactions of lipopolysaccharides from serogroups A,B,X, and Y Neisseria meningitidis. Can J Biochem. 1973 Oct;51(10):1347–1354. doi: 10.1139/o73-178. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Brandt B. L., Artenstein M. S. Antigenic specificity of bactericidal antibodies in antisera to Neisseria meningitidis. J Infect Dis. 1973 Apr;127(4):378–387. doi: 10.1093/infdis/127.4.378. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Wyle F. A., Formal S. B. Bactericidal antibody assay using 14 C-labeled Neisseria meningitidis. Proc Soc Exp Biol Med. 1972 Apr;139(4):1175–1180. doi: 10.3181/00379727-139-36324. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Limjuco G. A., Karkhanis Y. D., Zeltner J. Y., Maigetter R. Z., King J. J., Carlo D. J. Studies on the chemical composition of lipopolysaccharide from Neisseria meningitidis group B. J Gen Microbiol. 1978 Feb;104(2):187–191. doi: 10.1099/00221287-104-2-187. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977 May;16(2):471–475. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., MacGregor R. R., Beaty H. N. Bactericidal antibody after colonization with Neisseria meningitidis. J Infect Dis. 1973 Jan;127(1):56–62. doi: 10.1093/infdis/127.1.56. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


