Abstract
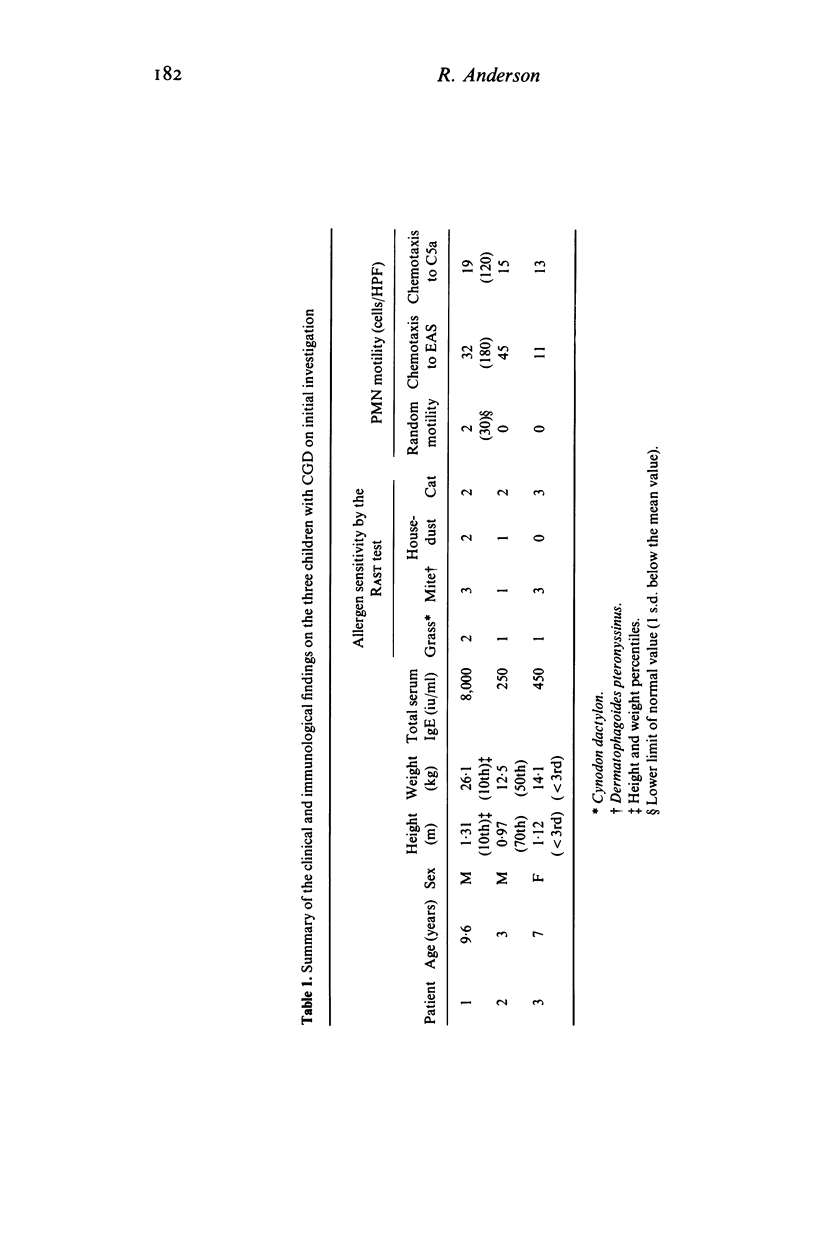
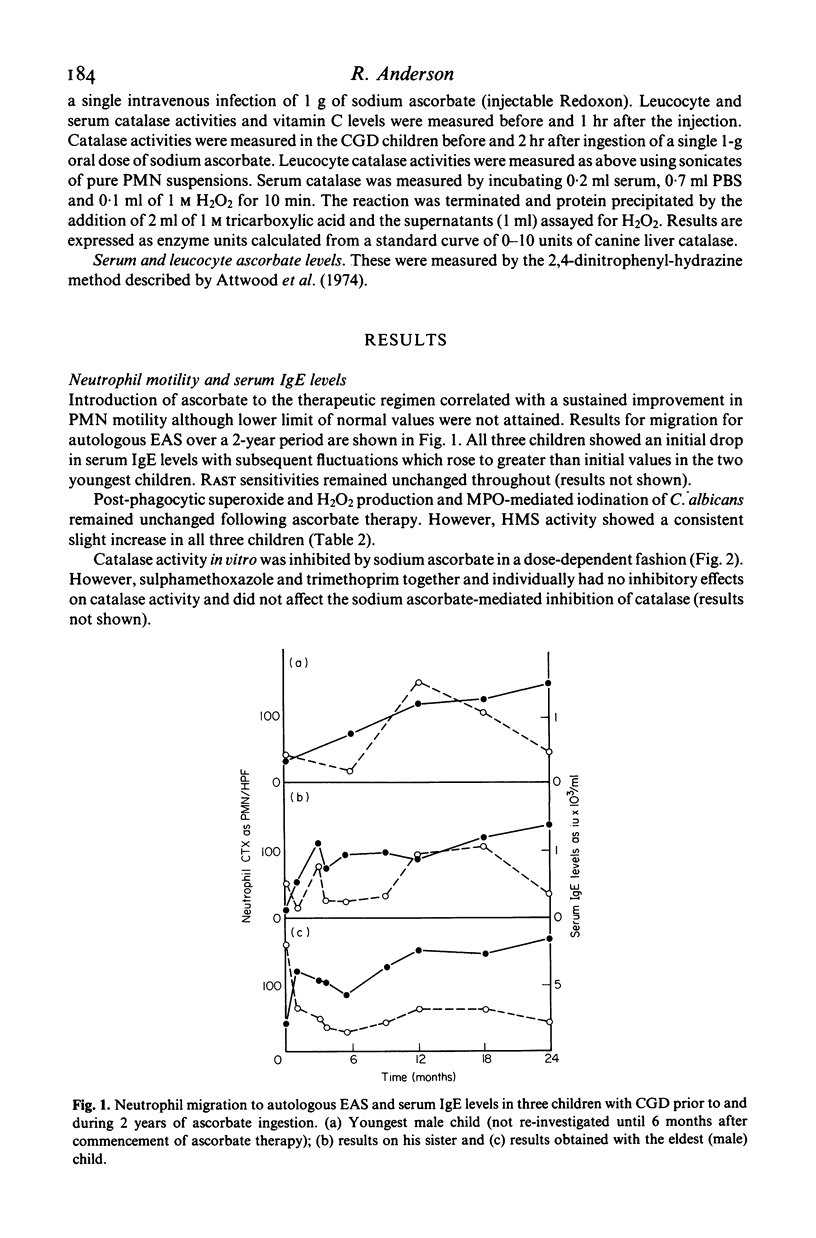
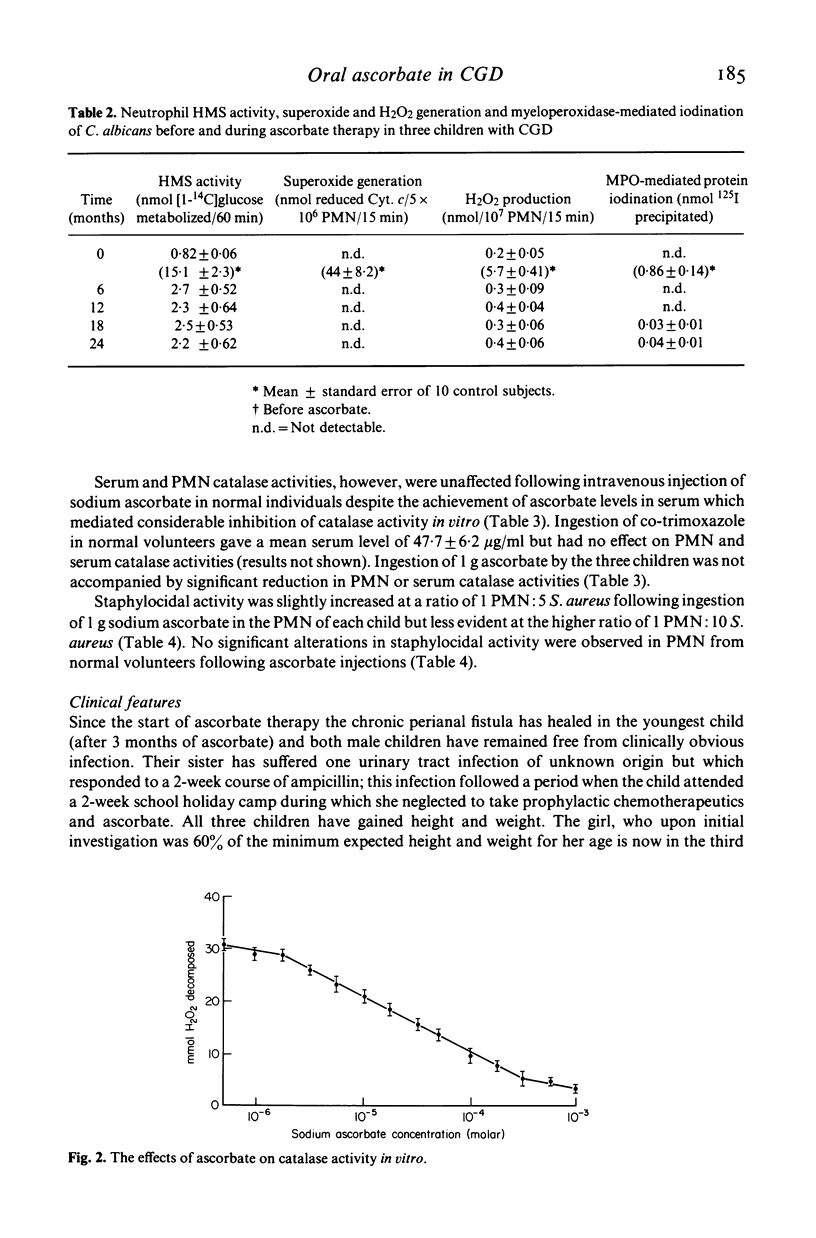
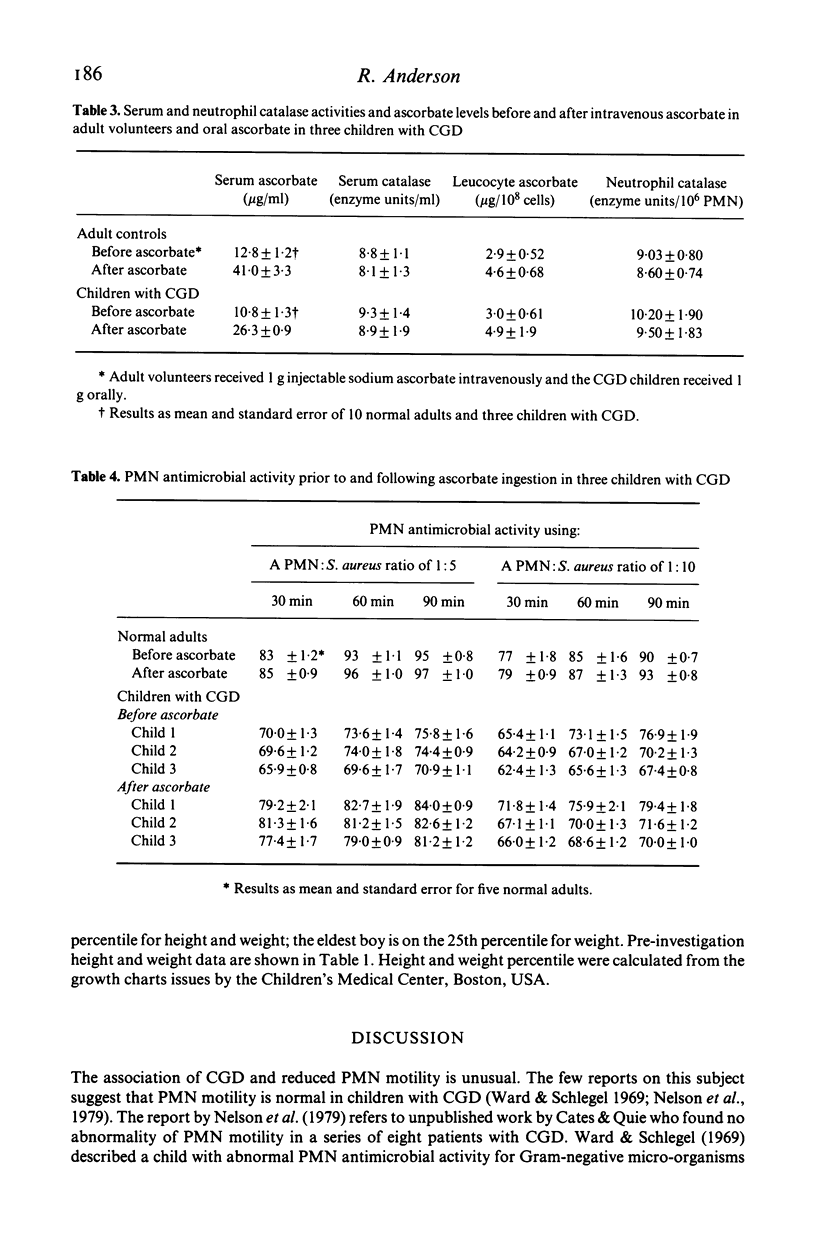
Two brothers and their sister with chronic granulomatous disease, elevated levels of serum IgE and defective neutrophil motility were treated with a single oral daily dose of 1 g sodium ascorbate as a supplement to prophylactic trimethoprim--sulphamethoxazole therapy for 2 years. Laboratory tests of neutrophil functions were performed prior to ascorbate therapy and repeated at 1-monthly intervals for 6 months and at 6-monthly intervals thereafter. Introduction of ascorbate to the therapeutic regimen was accompanied by slight increases in neutrophil hexose monophosphate shunt activity and staphylocidal activity and good improvement of neutrophil motility in all three children. The improved staphylocidal activity was not due to ascorbate-mediated inhibition of neutrophil or serum catalase activities or to detectable increases in superoxide and H2O2 production or activity of the MPO/H2O2/halide system. Both male children have remained free from obvious infection since ascorbate was added to their therapeutic regimen; their sister has experienced one urinary tract infection during a period when treatment with prophylactic co-trimoxazole and ascorbate was inadvertently stopped. All three children have gained weight.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Dittrich O. C. Effects of ascorbate on leucocytes: Part IV. Increased neutrophil function and clinical improvement after oral ascorbate in 2 patients with chronic granulomatous disease. S Afr Med J. 1979 Sep 1;56(12):476–480. [PubMed] [Google Scholar]
- Anderson R., Oosthuizen R., Maritz R., Theron A., Van Rensburg A. J. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr. 1980 Jan;33(1):71–76. doi: 10.1093/ajcn/33.1.71. [DOI] [PubMed] [Google Scholar]
- Anderson R., Oosthuizen R., Theron A., Van Rensburg A. J. The in vitro evaluation of certain neutrophil and lymphocyte functions following the ingestion of 150 mg oral dose of levamisole: assessment of the extent and duration of stimulation of neutrophil chemotaxis, protein iodination and lymphocyte transformation. Clin Exp Immunol. 1979 Mar;35(3):478–483. [PMC free article] [PubMed] [Google Scholar]
- Attwood E. C., Robey E. D., Ross J., Bradley F., Kramer J. J. Determination of platelet and leucocyte vitamin C and the levels found in normal subjects. Clin Chim Acta. 1974 Jul 15;54(1):95–105. doi: 10.1016/0009-8981(74)90047-3. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Watanabe A. M., Rister M., Besch H. R., Jr, Allen J., Baehner R. L. Correction of leukocyte function in Chediak-Higashi syndrome by ascorbate. N Engl J Med. 1976 Nov 4;295(19):1041–1045. doi: 10.1056/NEJM197611042951904. [DOI] [PubMed] [Google Scholar]
- Cooper M. R., McCall C. E., Dechatelet L. R. Stimulation of leukocyte hexose monophosphate shunt activity by ascorbic Acid. Infect Immun. 1971 Jun;3(6):851–853. doi: 10.1128/iai.3.6.851-853.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R., McCall C. E. Stimulation of the hexose monophosphate shunt in human neutrophils by ascorbic acid: mechanism of action. Antimicrob Agents Chemother. 1972 Jan;1(1):12–16. doi: 10.1128/aac.1.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERICSSON Y., LUNDBECK H. Antimicrobial effect in vitro of the ascorbic acid oxidation. I. Effect on bacteria, fungi and viruses in pure cultures. Acta Pathol Microbiol Scand. 1955;37(6):493–506. doi: 10.1111/j.1699-0463.1955.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E. L., Laxdal T., Quie P. G. Studies of polymorphonuclear leukocytes from patients with chronic granulomatous disease of childhood: bactericidal capacity for streptococci. Pediatrics. 1968 Mar;41(3):591–599. [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E. Killing and lysis of gram-negative bacteria through the synergistic effect of hydrogen peroxide, ascorbic acid, and lysozyme. J Bacteriol. 1969 Jun;98(3):949–955. doi: 10.1128/jb.98.3.949-955.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. G., Baum J. Polymorphonuclear leucocyte chemotaxis in patients with bacterial infections. Br Med J. 1971 Sep 11;3(5775):617–619. doi: 10.1136/bmj.3.5775.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Herron M., Simmons R. L., Quie P. G. Chemotactic deactivation of human neutrophils: possible relationship to stimulation of oxidative metabolism. Infect Immun. 1979 Feb;23(2):282–286. doi: 10.1128/iai.23.2.282-286.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C. W. Studies on ascorbic acid. I. Factors influencing the ascorbate-mediated inhibition of catalase. Biochemistry. 1967 Oct;6(10):2995–3000. doi: 10.1021/bi00862a004. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A. K. Colorimetric assay of catalase. Anal Biochem. 1972 Jun;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Root R. K., Vaughan M. Phagocytosis in chronic granulomatous disease and the Chediak-Higashi syndrome. N Engl J Med. 1972 Jan 20;286(3):120–123. doi: 10.1056/NEJM197201202860302. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Schlegel R. J. Impaired leucotactic responsiveness in a child with recurrent infections. Lancet. 1969 Aug 16;2(7616):344–347. doi: 10.1016/s0140-6736(69)92699-3. [DOI] [PubMed] [Google Scholar]


