Abstract
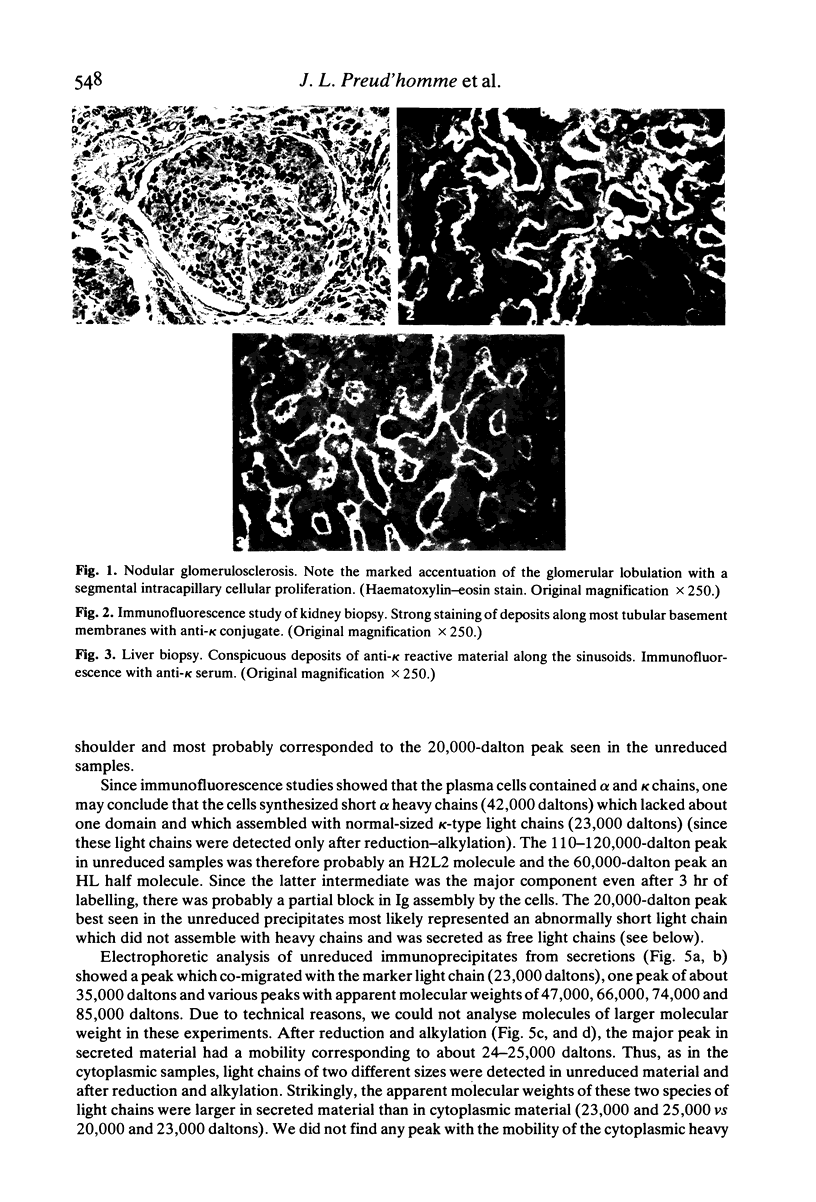
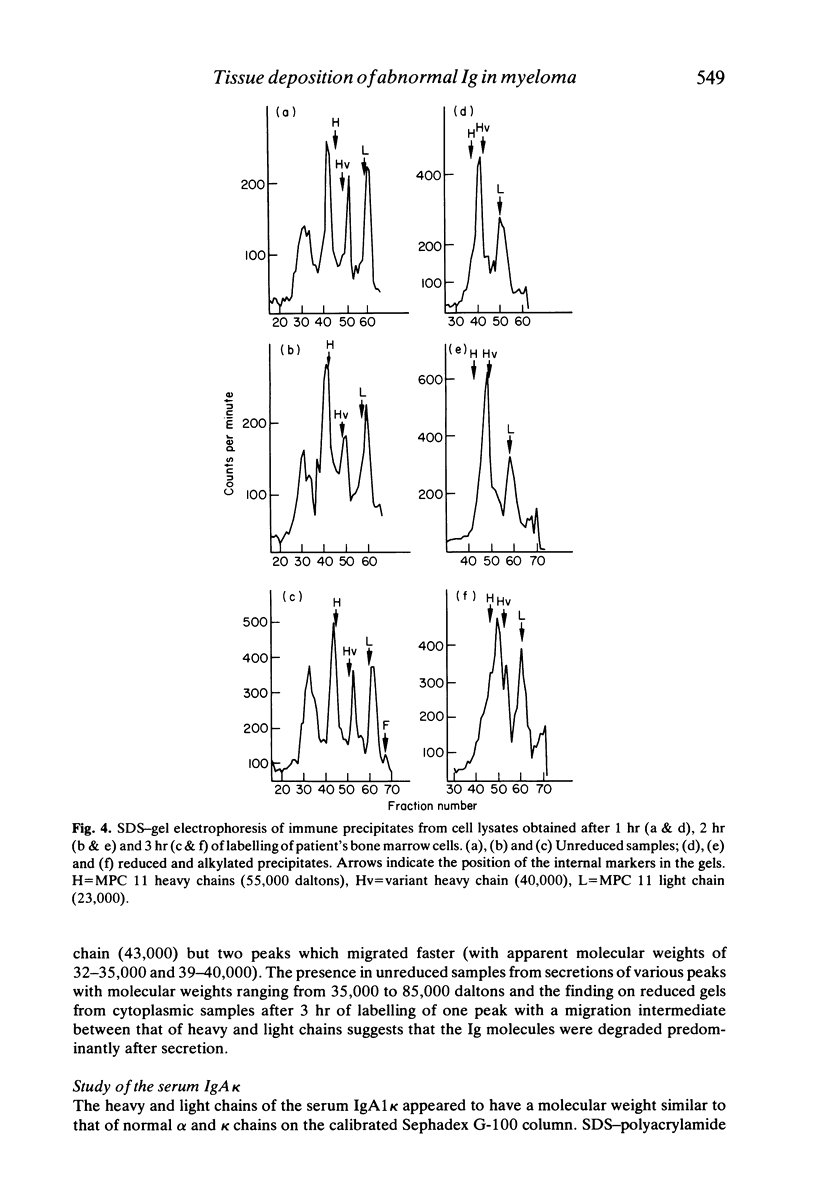
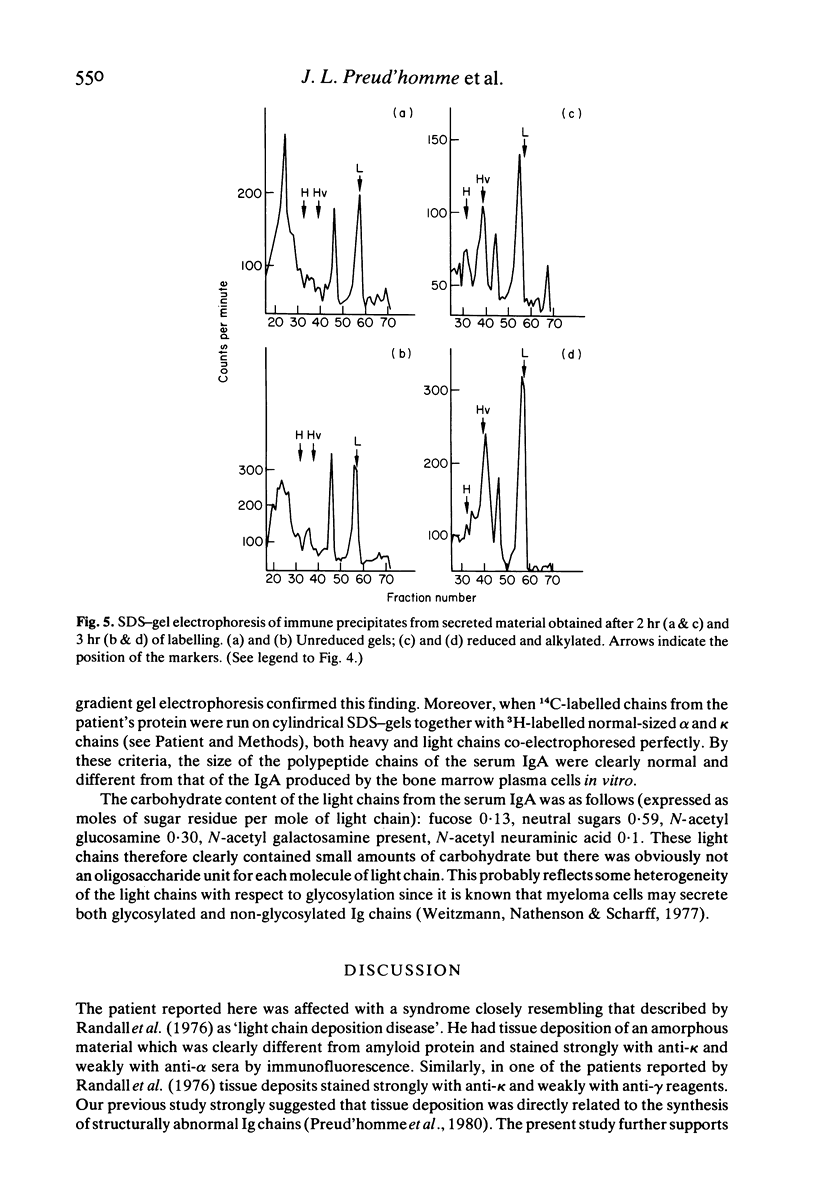
In a patient treated for IgA kappa myeloma, bone marrow relapse and a sharp drop in the serum IgA level paralleled tissue deposition of non-amyloid material reactive with anti-kappa anti-alpha sera in immunofluorescence studies of kidney and liver biopsies. Clinical manifestations were progressive renal failure with nephrotic syndrome, with both tubular and glomerular lesions (including nodular glomerulosclerosis), hepatomegaly, cardiac and neurological symptoms. Biosynthesis experiments showed the production of alpha chains diminished in length by about one domain which were rapidly degraded predominantly after secretion and of two species of light chains; normal-sized light chains which assembled with alpha chains and abnormally short ones which were secreted as free light chains. The apparent molecular weight of the light chains was larger in secretions than in cytoplasmic extracts, suggesting their glycosylation. These results suggest a causal relationship between tissue deposition and production of abnormal immunoglobulins by a variant clone, the emergence of which was possibly induced by Melphalan therapy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaufils M., Morel-Maroger L. Pathogenesis of renal disease in monoclonal gammopathies: current concepts. Nephron. 1978;20(3):125–131. doi: 10.1159/000181210. [DOI] [PubMed] [Google Scholar]
- Birshtein B. K., Preud'homme J. L., Scharff M. D. Variants of mouse myeloma cells that produce short immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3478–3482. doi: 10.1073/pnas.71.9.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver F. A., Hilschmann N. The primary structure of a monoclonal human lambda-type immunoglobulin L-chain of subgroup II (Bence-Jones protein NEI). Eur J Biochem. 1972 Mar 15;26(1):10–32. doi: 10.1111/j.1432-1033.1972.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Herf S., Pohl S. L., Sturgill B., Bolton W. K. An evaluation of diabetic and pseudodiabetic glomerulosclerosis. Am J Med. 1979 Jun;66(6):1040–1045. doi: 10.1016/0002-9343(79)90462-5. [DOI] [PubMed] [Google Scholar]
- Hobbs J. R. Immunocytoma o' mice an' men. Br Med J. 1971 Apr 10;2(5753):67–72. doi: 10.1136/bmj.2.5753.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel-Maroger L., Basch A., Danon F., Verroust P., Richet G. Pathology of the kidney in Waldenström's macroglobulinemia. Study of sixteen cases. N Engl J Med. 1970 Jul 16;283(3):123–129. doi: 10.1056/NEJM197007162830304. [DOI] [PubMed] [Google Scholar]
- Olsen S. Mesangial thickening and nodular glomerular sclerosis in diabetes mellitus and other diseases. Acta Pathol Microbiol Scand Suppl. 1972;233:203–216. [PubMed] [Google Scholar]
- Prend'homme J. L., Buxbaum J., Scharff M. D. Mutagenesis of mouse myeloma cells with 'Melphalan'. Nature. 1973 Oct 12;245(5424):320–322. doi: 10.1038/245320a0. [DOI] [PubMed] [Google Scholar]
- Preud'Homme J. L., Hurez D., Danon F., Brouet J. C., Seligmann M. Intracytoplasmic and surface-bound immunoglobulins in "nonsecretory" and Bence-Jones myeloma. Clin Exp Immunol. 1976 Sep;25(3):428–436. [PMC free article] [PubMed] [Google Scholar]
- Preud'homme J. L., Klein M., Labaume S., Seligmann M. Idiotype-bearing and antigen-binding receptors produced by blood T lymphocytes in a case of human myeloma. Eur J Immunol. 1977 Dec;7(12):840–846. doi: 10.1002/eji.1830071204. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Immunoglobulins on the surface of lymphoid cells in Waldenström's macroglobulinemia. J Clin Invest. 1972 Mar;51(3):701–705. doi: 10.1172/JCI106858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Williamson W. C., Jr, Mullinax F., Tung M. Y., Still W. J. Manifestations of systemic light chain deposition. Am J Med. 1976 Feb;60(2):293–299. doi: 10.1016/0002-9343(76)90440-x. [DOI] [PubMed] [Google Scholar]
- Sox H. C., Jr, Hood L. Attachment of carbohydrate to the variable region of myeloma immunoglobulin light chains. Proc Natl Acad Sci U S A. 1970 Jul;66(3):975–982. doi: 10.1073/pnas.66.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Abel C. A., Fishkin B. G., Grey H. M. Localization of the carbohydrate within the variable region of light and heavy chains of human gamma g myeloma proteins. Biochemistry. 1970 Oct 13;9(21):4217–4223. doi: 10.1021/bi00823a025. [DOI] [PubMed] [Google Scholar]
- Sølling K., Sølling J., Jacobsen N. O., Thomsen O. F. Nonsecretory myeloma associated with nodular glomerulosclerosis. Acta Med Scand. 1980;207(1-2):137–143. doi: 10.1111/j.0954-6820.1980.tb09693.x. [DOI] [PubMed] [Google Scholar]
- Weitzman S., Nathenson S. G., Scharff M. D. Abnormalities in the glycosylation of immunoglobulin heavy chain and an h-2 transplantation antigen in a mouse myeloma mutant. Cell. 1977 Apr;10(4):679–687. doi: 10.1016/0092-8674(77)90101-5. [DOI] [PubMed] [Google Scholar]
- Weitzman S., Palmer L., Grennon M. Serum decay and placental transport of a mutant mouse myeloma immunoglobulin with defective polypeptide and oligosaccharide structure. J Immunol. 1979 Jan;122(1):12–18. [PubMed] [Google Scholar]



