Abstract
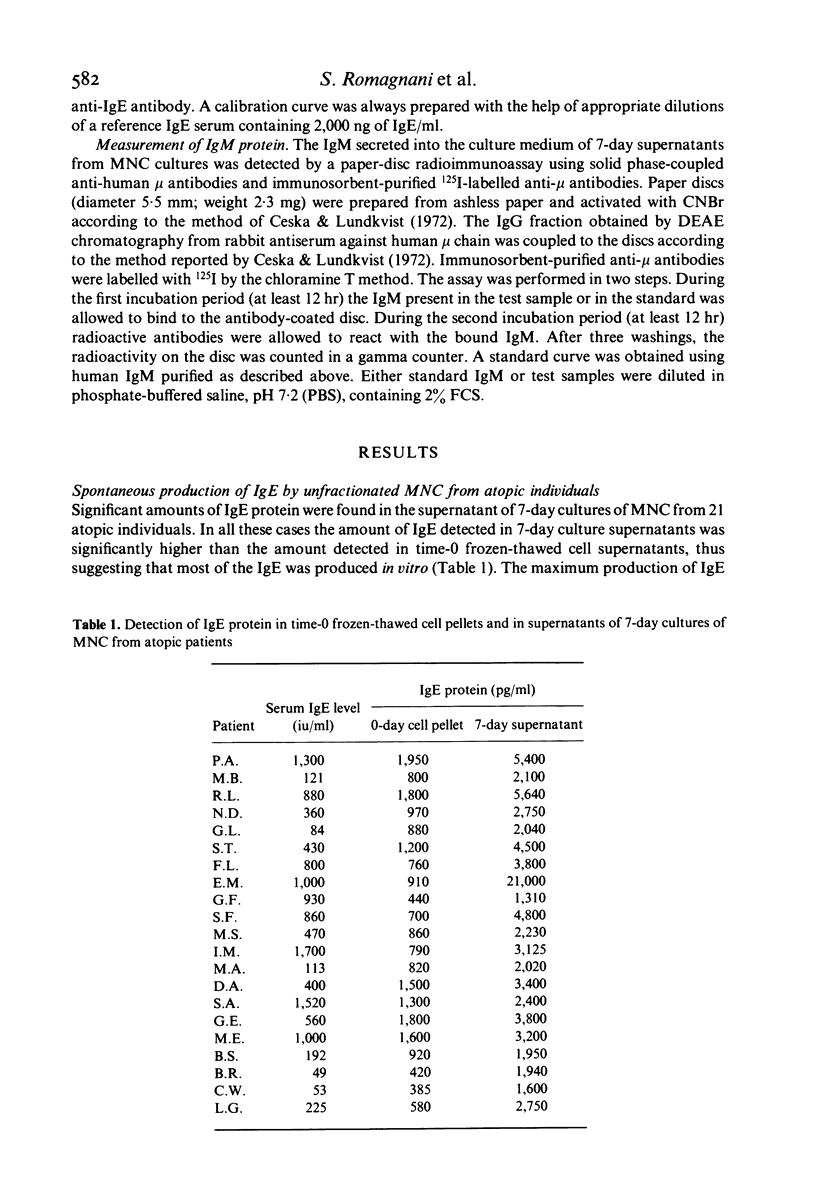
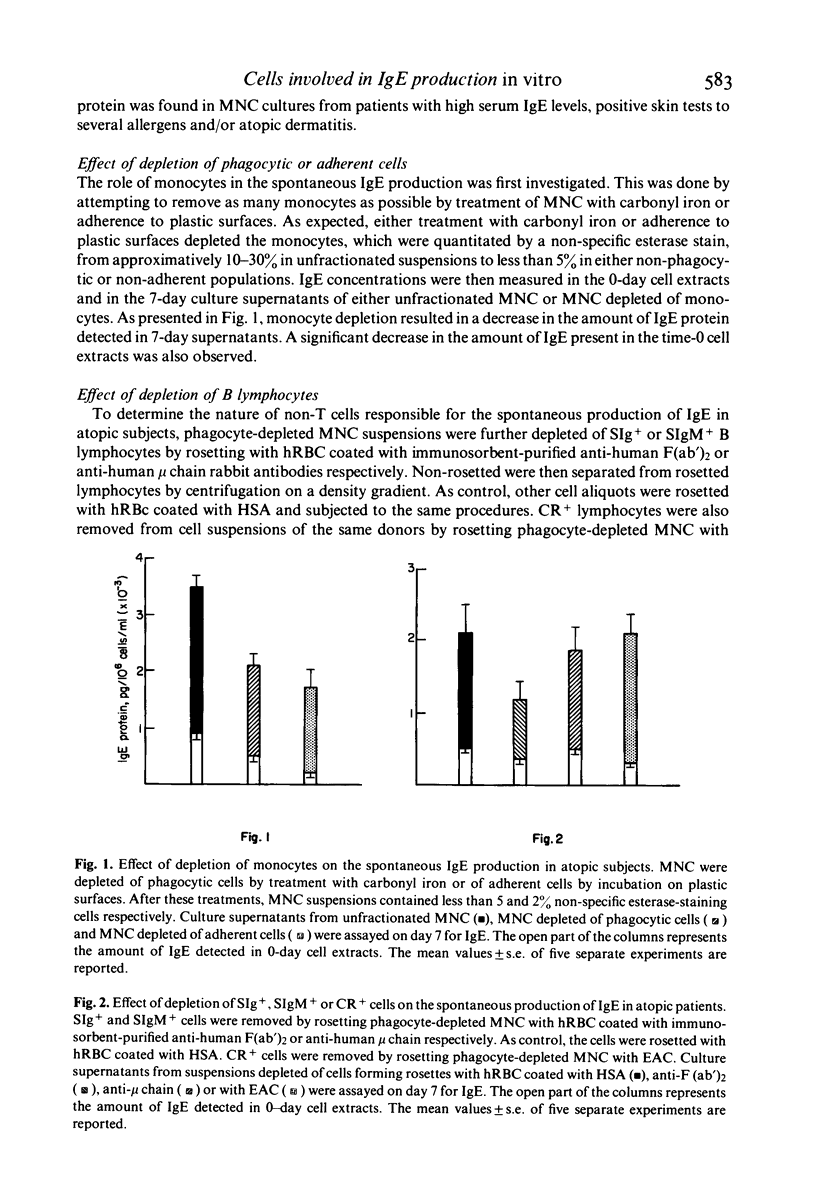
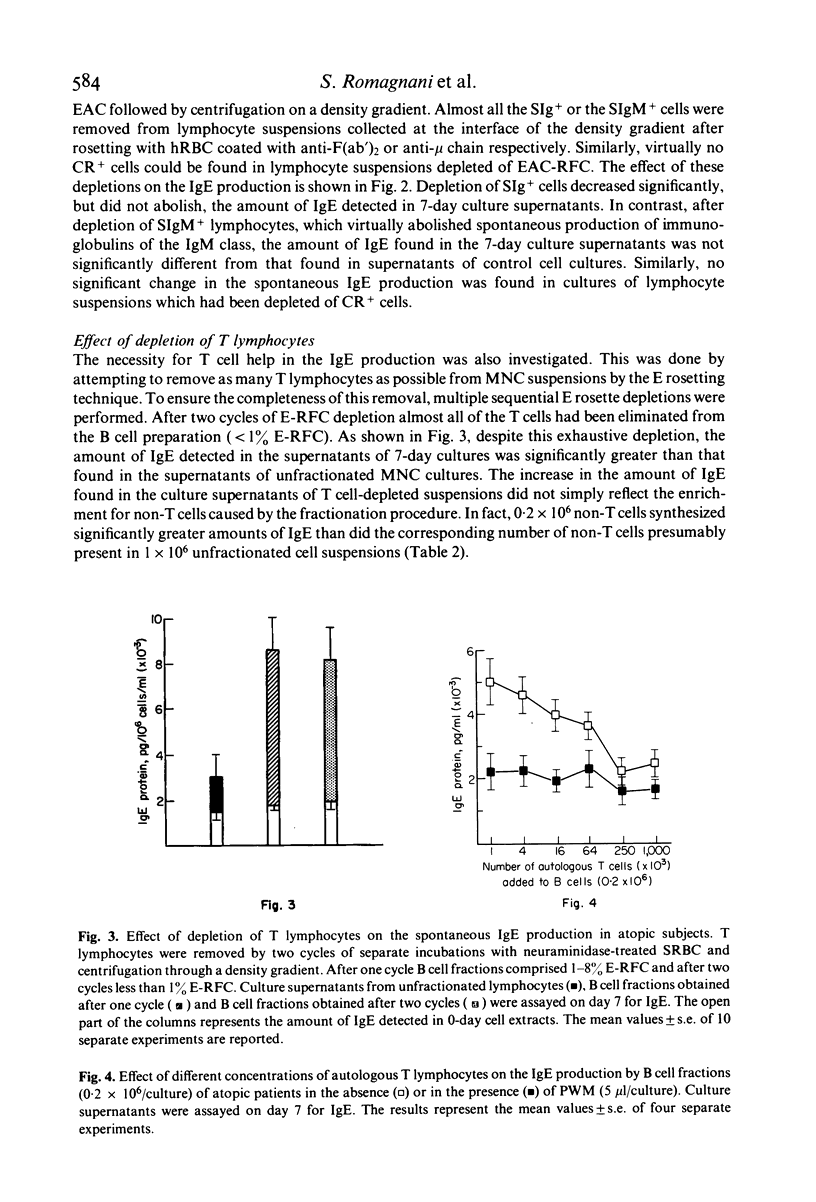
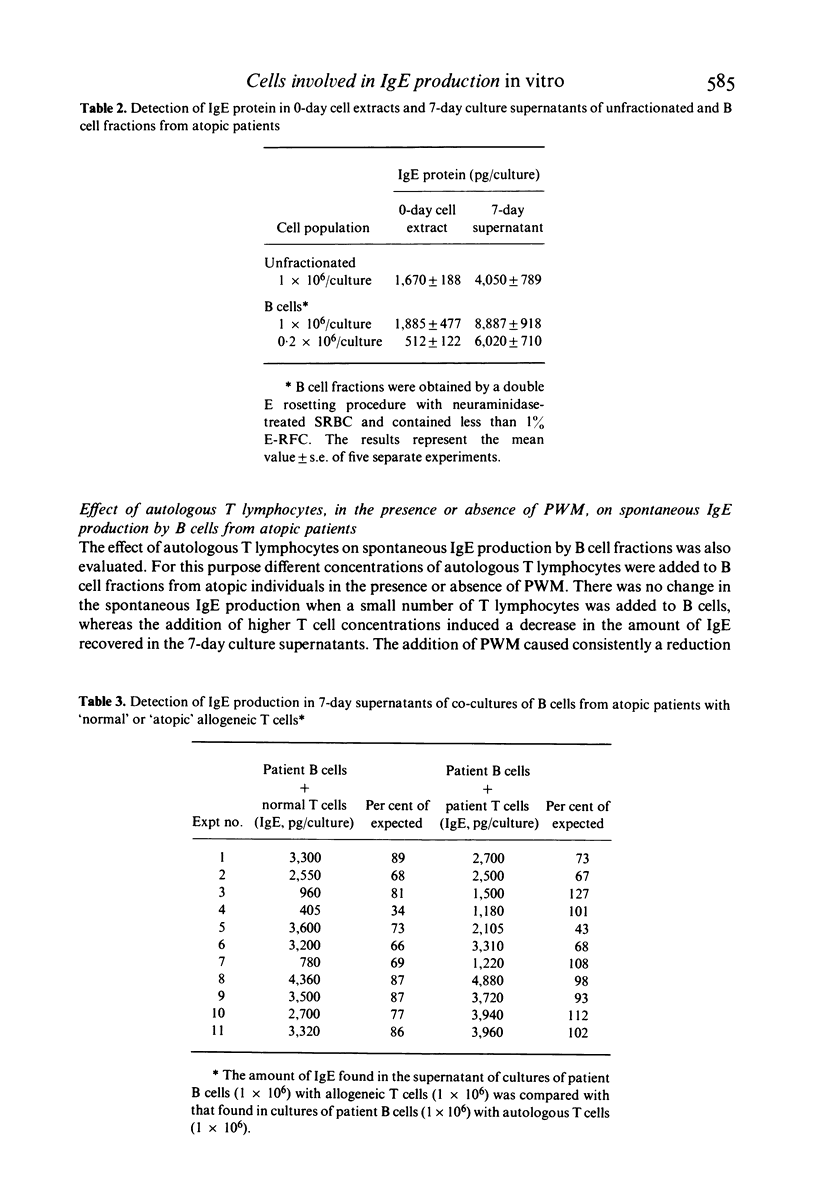
Spontaneous IgE production in vitro was investigated in 7-day cultures of unfractionated mononuclear cells (MNC) and MNC subpopulations from atopic patients. Depletion of either phagocytic or adherent cells decreased the amount of IgE detectable in 7-day culture supernatants, but this decrease was due, at least in part, to a loss of cytophilic IgE. Depletion of immunoglobulin-bearing cells (SIg+) reduced significantly but did not abolish the spontaneous IgE production in vitro. On the other hand, depletion of IgM-bearing lymphocytes (SIgM+), which virtually abolished the production of immunoglobulins of the IgM class, did not change significantly the spontaneous production of IgE. Similarly, no change in the spontaneous production of IgE was found when lymphocyte suspensions were depleted of complement receptor-bearing cells (CR+). In contrast, spontaneous IgE production was significantly increased by depletion of T lymphocytes and this increase did not simply reflect the enrichment for IgE-producing cells caused by the fractionation procedure. No significant change in the spontaneous IgE production was found when small numbers of autologous T lymphocytes were added to B cell fractions, whereas the addition of higher concentrations of autologous T cells induced a marked inhibition of the spontaneous IgE production. On the other hand, the addition in culture of pokeweed mitogen (PWM) resulted in a marked reduction of the spontaneous IgE production by B cells, also in the presence of small concentrations of autologous T lymphocytes. Normal T cells were consistently effective in inducing a partial inhibition of the spontaneous IgE production by B cells from atopic patients, whereas T cells from a noticeable proportion of atopic patients were not. These data suggest that MNC responsible for the spontaneous IgE production in atopic subjects are SIgM- and CR-deficient well-differentiated lymphocytes which probably represent the result of an activation which has occurred in vivo. However, this spontaneous IgE production can still be influenced by in vitro manipulation, such as variations in T–B cell ratios or addition of PWM. The results here reported also indicate that normal T cells are generally more effective than T cells from atopic patients in regulating the activity of spontaneous IgE-producing cells present in the blood of atopic subjects.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceska M., Lundkvist U. A new and simple radioimmunoassay method for the determination of IgE. Immunochemistry. 1972 Oct;9(10):1021–1030. doi: 10.1016/0019-2791(72)90112-7. [DOI] [PubMed] [Google Scholar]
- Fiser P. M., Buckley R. H. Human IgE biosynthesis in vitro: studies with atopic and normal blood mononuclear cells and subpopulations. J Immunol. 1979 Oct;123(4):1788–1794. [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Gonzalez-Molina A., Spiegelberg H. L. A subpopulation of normal human peripheral B lymphcytes that bind IgE. J Clin Invest. 1977 Apr;59(4):616–624. doi: 10.1172/JCI108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R., Suszko I. M., Hsu C. C., Roberts M., Oh S. H. In vitro production of IgE by lymphocytes from a patient with hyperimmunoglobulinaemia E, eosinophilia and increased lymphocytes carrying surface IgE. Clin Exp Immunol. 1975 May;20(2):265–272. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Almerigogna F., Ricci M. Interaction of staphylococcal protein A with membrane components of IgM- and/or IgD-bearing lymphocytes from human tonsil. J Immunol. 1980 Apr;124(4):1620–1626. [PubMed] [Google Scholar]
- Romagnani S., Maggi E., Del Prete G. F., Troncone R., Ricci M. In vitro production of IgE by human peripheral blood mononuclear cells. I. Rate of IgE biosynthesis. Clin Exp Immunol. 1980 Oct;42(1):167–174. [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H. Stimulation and regulation of human IgE production in vitro using peripheral blood lymphocytes. Clin Immunol Immunopathol. 1979 Dec;14(4):474–488. doi: 10.1016/0090-1229(79)90100-4. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Melewicz F. M. Fc receptors specific for IgE on subpopulations of human lymphocytes and monocytes. Clin Immunol Immunopathol. 1980 Mar;15(3):424–433. doi: 10.1016/0090-1229(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Tjio A. H., Hull W. M., Gleich G. J. Production of human immunoglobulin E antibody in vitro. J Immunol. 1979 May;122(5):2131–2133. [PubMed] [Google Scholar]
- Yodoi J., Ishizaka K. Lymphocytes bearing Fc receptors for IgE. I. Presence of human and rat T lymphocytes with Fc epsilon receptors. J Immunol. 1979 Jun;122(6):2577–2583. [PubMed] [Google Scholar]


