Abstract
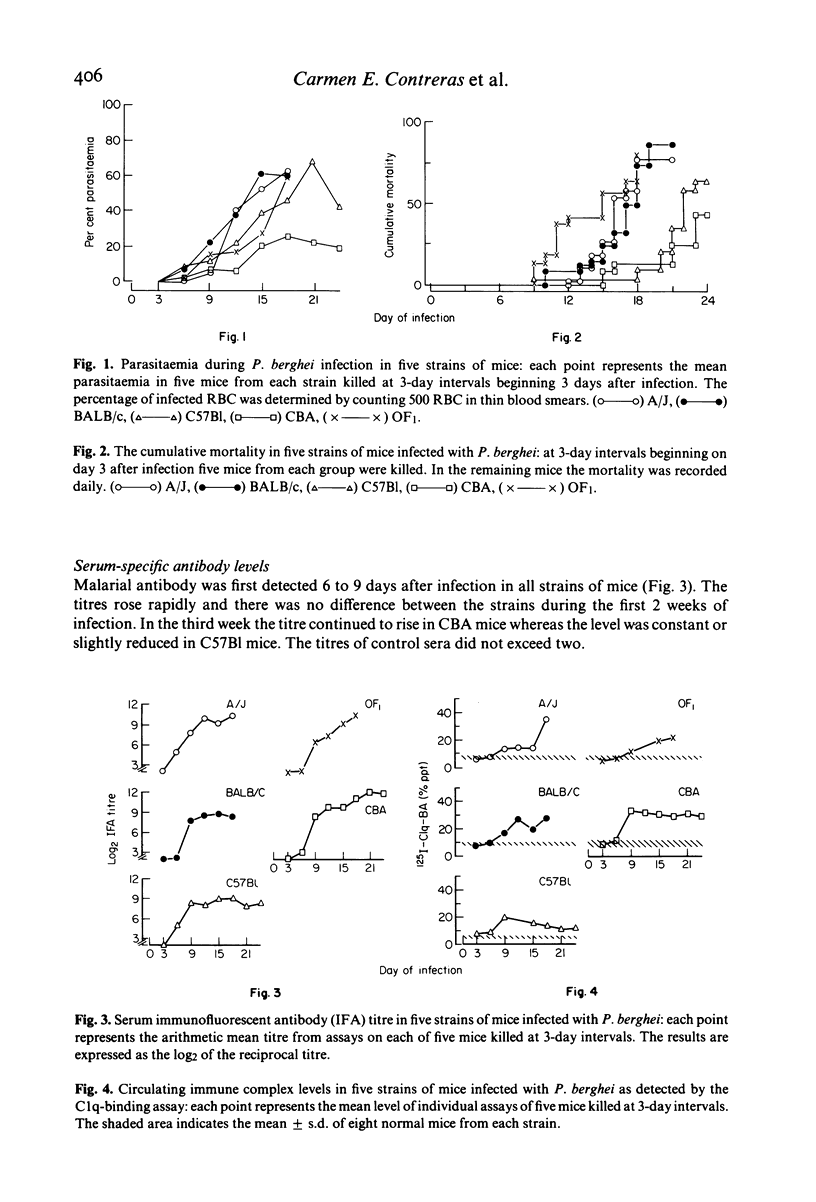
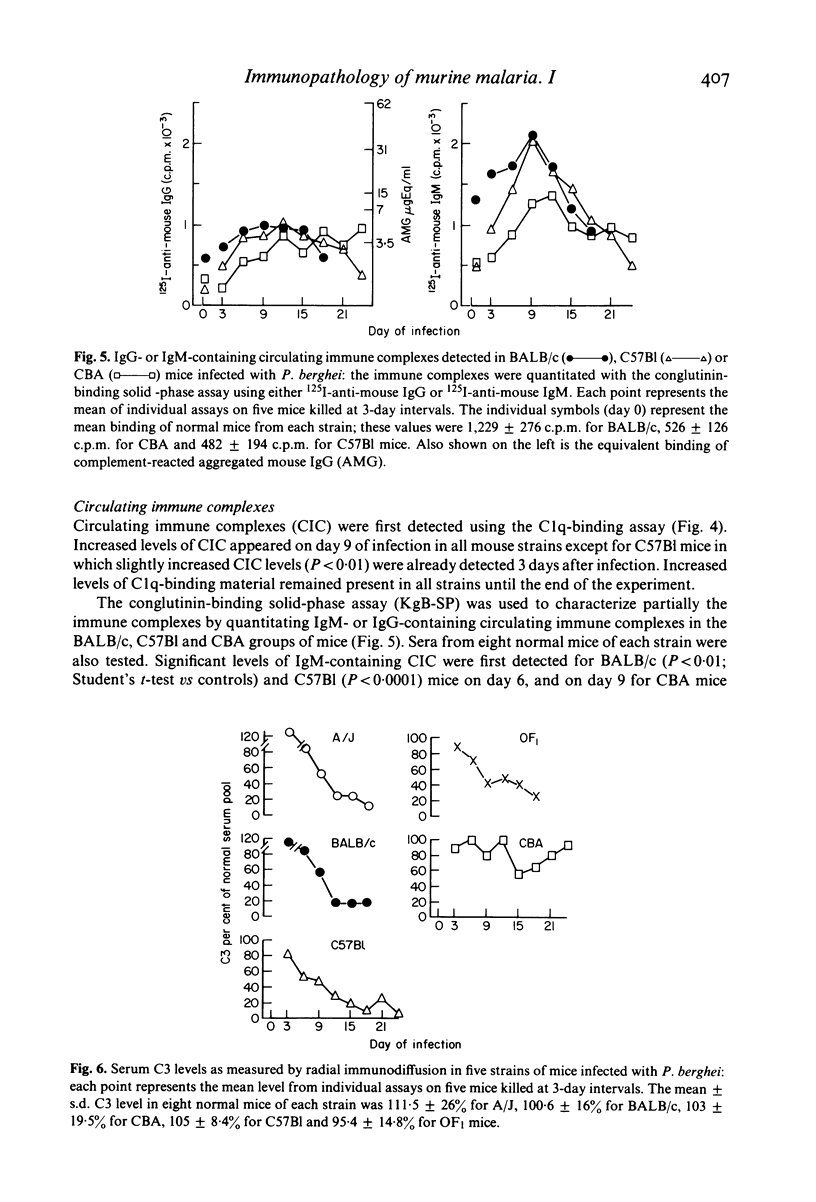
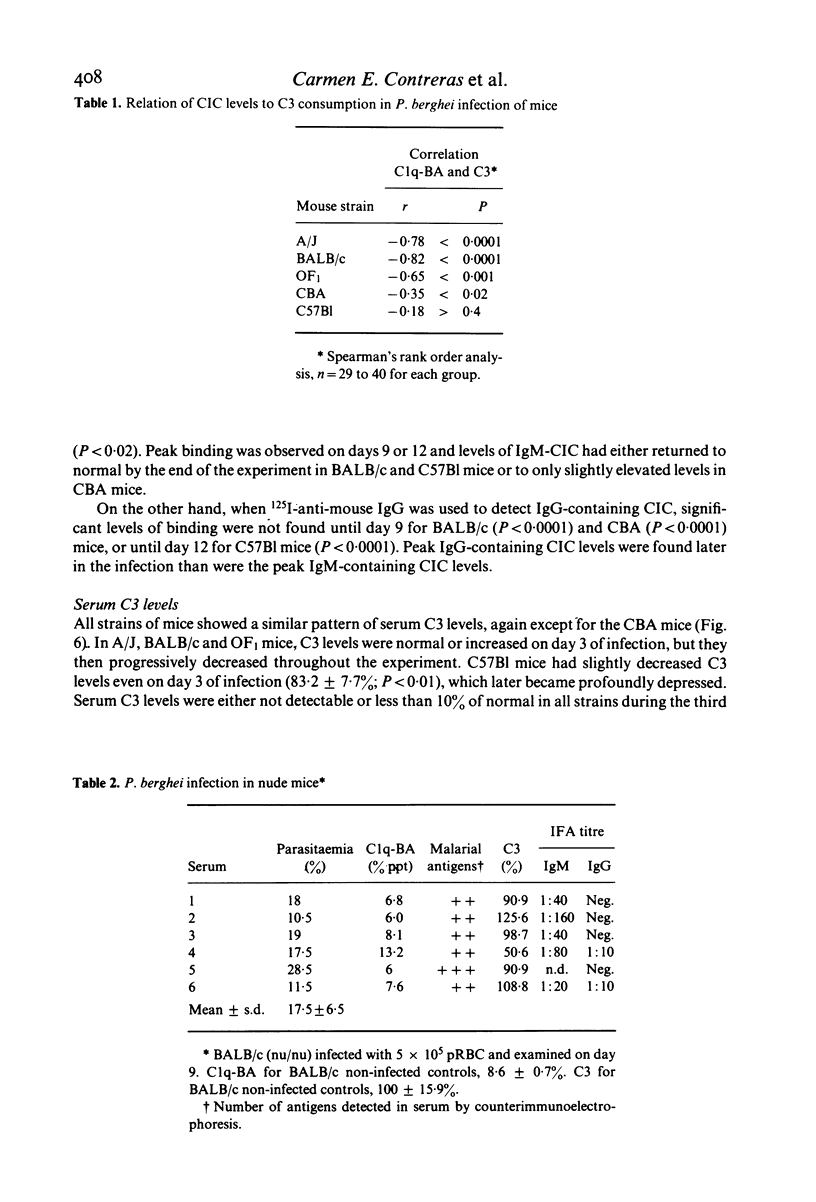
The development of circulating immune complexes was studied in mice of the BALB/c, A/J, OF1, CBA and C57B1 strains infected with P. berghei. Complexes were evaluated in relation to levels of parasitaemia, soluble antigen, specific antibody and C3. Susceptibility to infection was greatest in BALB/c, A/J and OF/a mice. The maximum parasitaemia was 30% in CBA and 70% in all other strains. Levels of soluble antigen paralleled those of parasitaemia. Specific antibody was detected in all strains, but the titre continued to rise throughout the infection only in CBA mice. Circulating immune complexes occurred in mice of all strains from day 6; the level fell after day 9 in C57B1 whereas it was maintained in CBA mice. The development of immune complexes was associated with marked depression of C3 levels in all except CBA mice, in which a transient reduction was followed by recovery. Partial characterization of the complexes showed that IgM-containing complexes appeared earliest and reached highest levels in BALB/c mice while in CBA mice, IgM complexes were found in lesser amounts and the level fell in late infection. IgG complexes rose throughout infection in CBA and fell in later stages in BALB/c and C57B1 mice. In nude BALB/c mice, immune complexes were usually not detectable and only low levels of antibody of IgM class were produced. Differences in mortality pattern could not be related to any single serological factor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Powell R. D. A study of the relationship between the content of adenosine triphosphate in human red cells and the course of falciparum malaria: a new system that may confer protection against malaria. Proc Natl Acad Sci U S A. 1965 Sep;54(3):741–745. doi: 10.1073/pnas.54.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Bossus A., Carpentier N. A., Lambert P. H. Solid-phase enzyme immunoassay or radioimmunoassay for the detection of immune complexes based on their recognition by conglutinin: conglutinin-binding test. A comparative study with 125I-labelled Clq binding and Raji-cell RIA tests. Clin Exp Immunol. 1977 Aug;29(2):342–354. [PMC free article] [PubMed] [Google Scholar]
- Eling W., van Zon A., Jerusalem C. The course of a Plasmodium berghei infection in six different mouse strains. Z Parasitenkd. 1977 Dec 13;54(1):29–45. doi: 10.1007/BF00380634. [DOI] [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Malaria infections in different strains of mice and their correlation with natural killer activity. Bull World Health Organ. 1979;57 (Suppl 1):231–238. [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J., KENDRICK L. P. Parasitemia and survival in inbred strains of mice infected with Plasmodium berghei. J Parasitol. 1957 Aug;43(4):413–419. [PubMed] [Google Scholar]
- GREENBERG J., KENDRICK L. P. Some characteristics of Plasmodium berghei passed within inbred strains of mice. J Parasitol. 1957 Aug;43(4):420–427. [PubMed] [Google Scholar]
- June C. H., Contreras C. E., Perrin L. H., Lambert P. H., Miescher P. A. Circulating and tissue-bound immune complex formation in murine malaria. J Immunol. 1979 Jun;122(6):2154–2161. [PubMed] [Google Scholar]
- June C. H., Contreras C. E., Perrin L. H., Lambert P. Improved detection of immune complexes in human and mouse serum using a microassay adaptation of the C1Q binding test. J Immunol Methods. 1979;31(1-2):23–29. doi: 10.1016/0022-1759(79)90282-5. [DOI] [PubMed] [Google Scholar]
- Mackey L. J., Hochmann A., June C. H., Contreras C. E., Lambert P. H. Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin Exp Immunol. 1980 Dec;42(3):412–420. [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Miller G. W., Nussenzweig V. A new complement function: solubilization of antigen-antibody aggregates. Proc Natl Acad Sci U S A. 1975 Feb;72(2):418–422. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel E M, Greenberg J, Jay G E, Coatney G R. Backcross Studies on the Genetics of Resistance to Malaria in Mice. Genetics. 1955 Sep;40(5):620–626. doi: 10.1093/genetics/40.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill P., Johnson G. D. Multispot immunofluorescence: a simple semi-automatic method of processing large numbers of tests. J Clin Pathol. 1970 Mar;23(2):185–187. doi: 10.1136/jcp.23.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz H. M. Counter-current immunoelectrophoresis for the demonstration of malarial antigens and antibodies in the sera of rats and mice. Trans R Soc Trop Med Hyg. 1975;69(1):88–90. doi: 10.1016/0035-9203(75)90015-2. [DOI] [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]


