Abstract
We have studied two patients, one with red cell aplasia and the other with neutropenia. Both showed lymphocytosis. In both cases, 90-100% of E rosetting cells were T cells as defined by the monoclonal antibodies UCHT1 and OKT3. The majority of these cells also carried the OKT8 suppressor/cytotoxic marker and were HLA-DR- and Fc gamma R-positive. In spite of the similarity of this phenotype to that reported for suppressor cells, these cells failed to suppress pokeweed mitogen-induced polyclonal Ig synthesis. Cells from both patients also failed to respond significantly to Con A and PHA. They were, however, unable to suppress the Con A responses of normal donors although cells from one patient were able to suppress completely a normal PHA response. These results demonstrate the existence of a genuine subset of T cells with Fc gamma receptors but suggest that not all such cells have typical suppressor function.
Full text
PDF


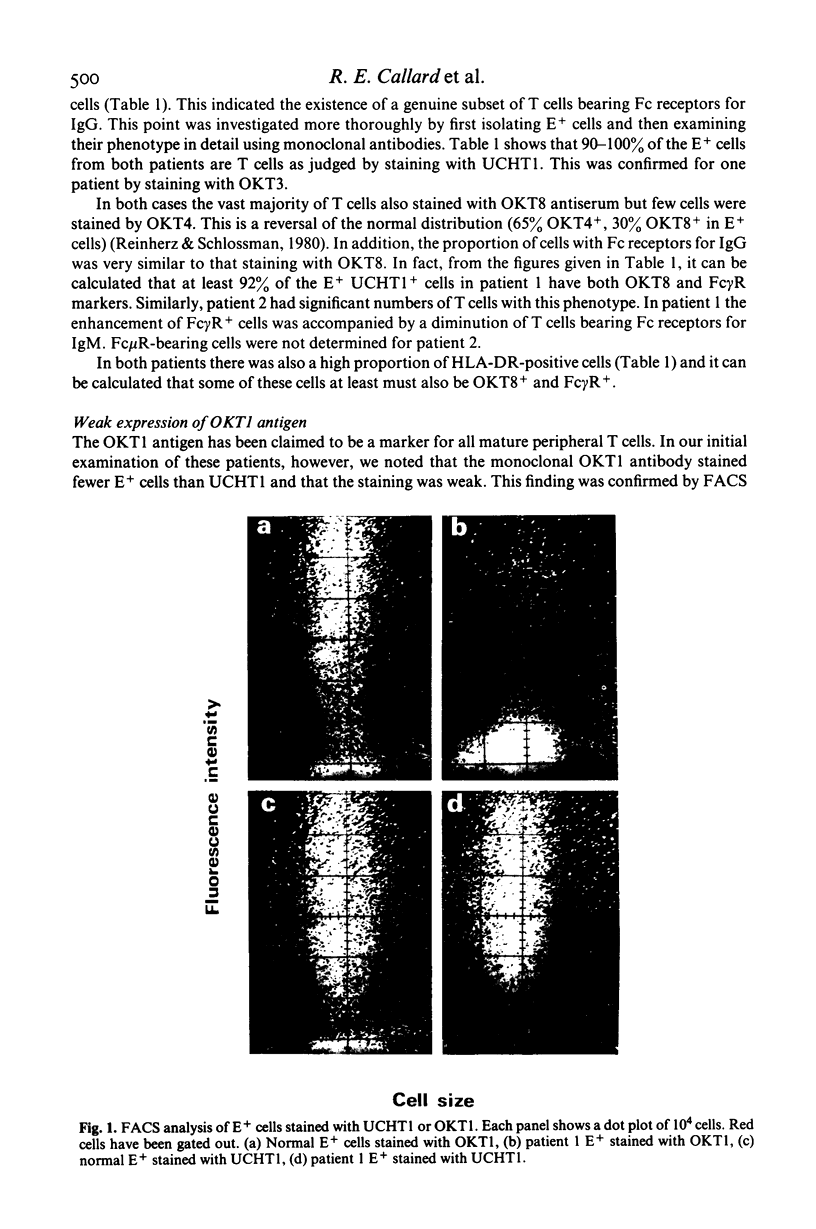
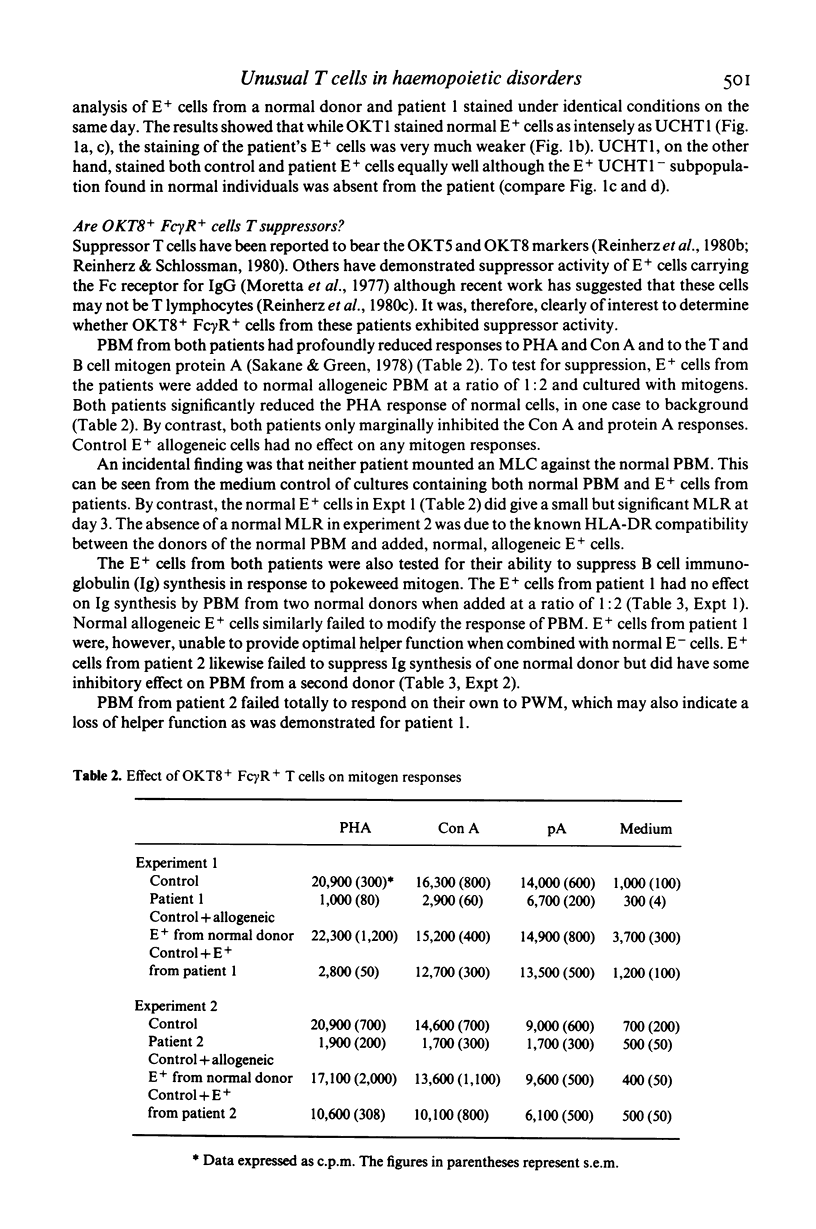
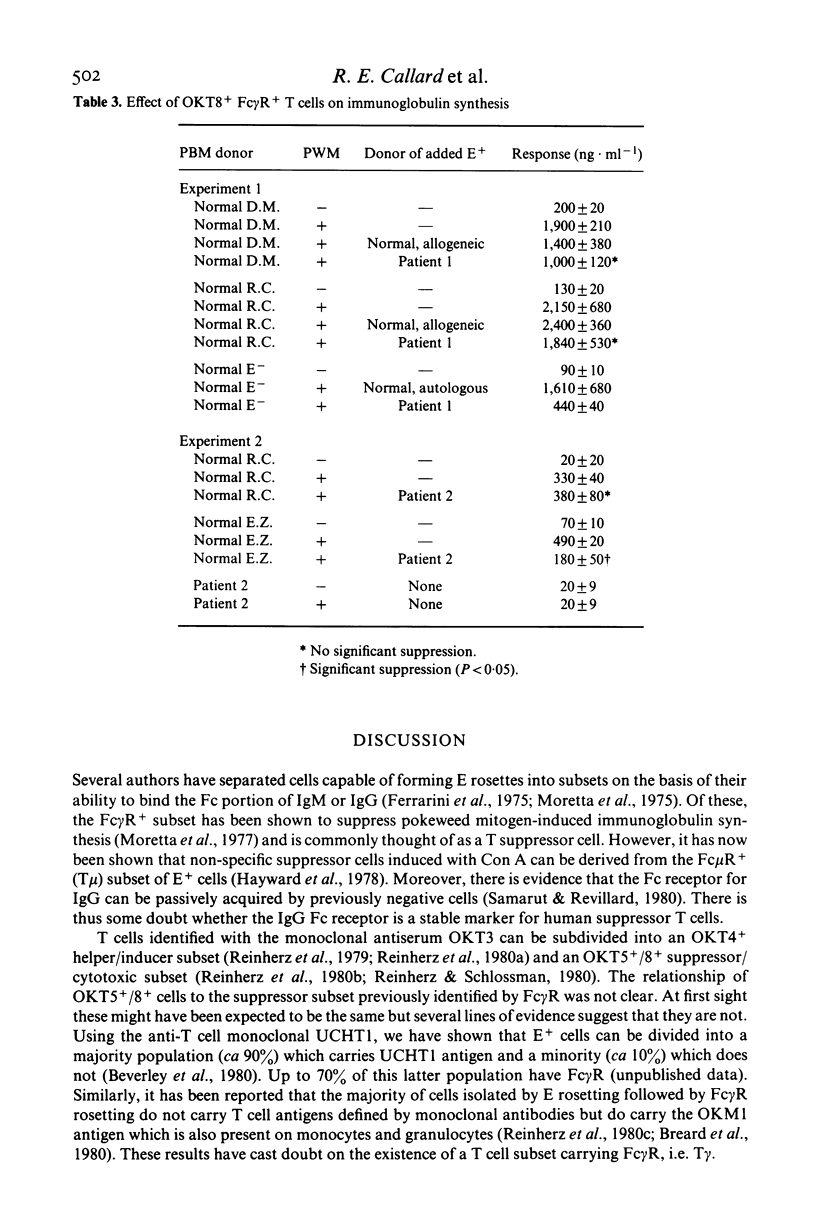
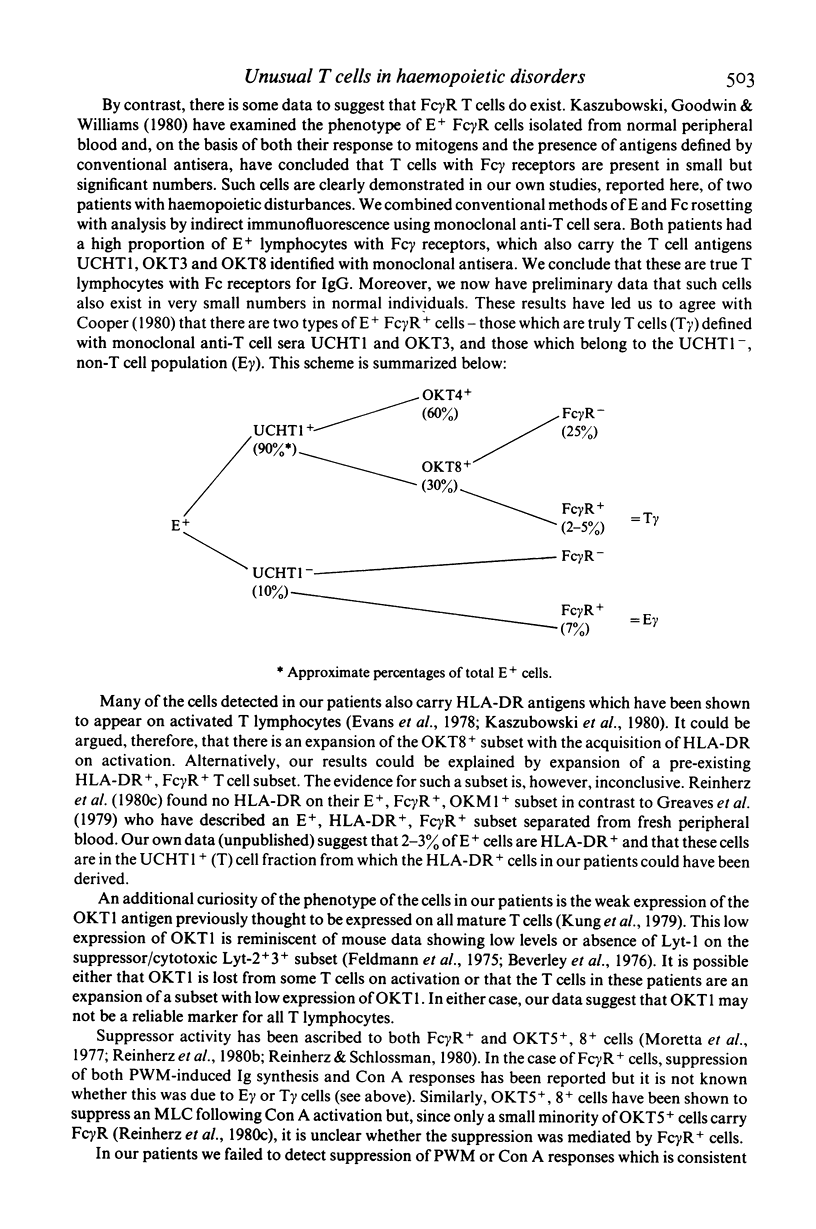


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. C., Woody J., Dunkley M., Feldmann M., McKenzie I. Separation of suppressor and killer T cells by surgace phenotype. Nature. 1976 Aug 5;262(5568):495–497. doi: 10.1038/262495a0. [DOI] [PubMed] [Google Scholar]
- Bom-van Noorloos A. A., Pegels H. G., van Oers R. H., Silberbusch J., Feltkamp-Vroom T. M., Goudsmit R., Zeijlemaker W. P., von dem Borne A. E., Melief C. J. Proliferation of T gamma cells with killer-cell activity in two patients with neutropenia and recurrent infections. N Engl J Med. 1980 Apr 24;302(17):933–937. doi: 10.1056/NEJM198004243021702. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Burns G. F., Cawley J. C., Barker C. R., Goldstone A. H., Hayhoe F. G. New evidence relating to the nature and origin of the hairy cell of leukaemic reticuloendotheliosis. Br J Haematol. 1977 May;36(1):71–84. doi: 10.1111/j.1365-2141.1977.tb05757.x. [DOI] [PubMed] [Google Scholar]
- Cooper M. D. Immunologic analysis of lymphoid tumors. N Engl J Med. 1980 Apr 24;302(17):964–965. doi: 10.1056/NEJM198004243021710. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Beverley P. C., Dunkley M., Kontiainen S. Different Ly antigen phenotypes of in vitro induced helper and suppressor cells. Nature. 1975 Dec 18;258(5536):614–616. doi: 10.1038/258614a0. [DOI] [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Verbi W., Festenstein H., Papasteriadis C., Jaraquemada D., Hayward A. "Ia-like" antigens on human T cells. Eur J Immunol. 1979 May;9(5):356–362. doi: 10.1002/eji.1830090504. [DOI] [PubMed] [Google Scholar]
- Hallberg T., Gurner B. W., Coombs R. R. Opsonic adherence of sensitized ox red cells to human lymphocytes as measured by rosette formation. Int Arch Allergy Appl Immunol. 1973;44(4):500–513. doi: 10.1159/000230956. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Schooley R. T., Grouse J. E., Payling-Wright C. R., Dolin R., Fauci A. S. Characterization of thymus-derived lymphocyte subsets in acute Epstein-Barr virus-induced infectious mononucleosis. J Immunol. 1979 Feb;122(2):699–702. [PubMed] [Google Scholar]
- Hayward A. R., Layward L., Lydyard P. M., Moretta L., Dagg M., Lawton A. R. Fc-receptor heterogeneity of human suppressor T cells. J Immunol. 1978 Jul;121(1):1–5. [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Kaszubowski P. A., Goodwin J. S., Williams R. C., Jr Ia antigen on the surface of a subfraction of T cells that bear Fc receptors for IgG. J Immunol. 1980 Mar;124(3):1075–1078. [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Breard J. M., Goldstein G., Schlossman S. F. T cell requirements for generation of helper factor(s) in man: analysis of the subsets involved. J Immunol. 1980 Apr;124(4):1883–1887. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Sakane T., Green I. Protein A from Staphylococcus aureus-a mitogen for human T lymphocytes and B lymphocytes but not L lymphocytes. J Immunol. 1978 Jan;120(1):302–311. [PubMed] [Google Scholar]
- Samarut C., Revillard J. P. Active and passive re-expression of Fc gamma receptors on human lymphocytes. Eur J Immunol. 1980 May;10(5):352–358. doi: 10.1002/eji.1830100507. [DOI] [PubMed] [Google Scholar]


