Abstract
Through direct interaction with the voltage-dependent anion channel (VDAC), proapoptotic Bcl-2 family members such as Bax and Bak induce apoptogenic mitochondrial cytochrome c release and membrane potential (Δψ) loss in isolated mitochondria. Using isolated mitochondria, we showed that Bid and Bik, BH3-only proteins from the Bcl-2 family, induced cytochrome c release but not Δψ loss. Unlike Bax/Bak, the cytochrome c release induced by Bid/Bik was Ca2+-independent, cyclosporin A-insensitive, and respiration-independent. Furthermore, in contrast to Bax/Bak, Bid/Bik neither interacted with VDAC nor directly affected the VDAC activity in liposomes. Consistently, Bid/Bik induced apoptosis without Δψ loss, whereas Bax induced apoptosis with Δψ loss. These findings indicated the involvement of a different mechanism in BH3-only, protein-induced apoptogenic cytochrome c release.
The Bcl-2 family of proteins is a well characterized regulator of apoptosis. It consists of the following three distinct subfamilies: (i) antiapoptotic members such as Bcl-2 and Bcl-xL with sequence homology at BH (Bcl-2 homology)1, BH2, BH3, and BH4 domains, (ii) proapoptotic members such as Bax and Bak with sequence homology at BH1, BH2, and BH3, and (iii) proapoptotic proteins that share homology only at the BH3 domain (BH3-only proteins) such as Bid, Bik, and Bim (1, 2).
Recent evidence has shown that the mitochondria play a crucial role in apoptosis (1–3) by releasing apoptogenic factors such as cytochrome c (4–6) and apoptosis-inducing factor (AIF) (7) from the intermembrane space into the cytoplasm. Once in the cytoplasm, cytochrome c activates a major apical caspase, caspase-9, in concert with Apaf-1 and deoxyATP (or ATP), and, subsequently, caspase-9 activates an effector caspase, caspase-3 (8, 9). AIF has been reported to induce apoptotic nuclear changes in a caspase-independent manner (7). It has been shown that Bcl-2 family proteins regulate apoptotic changes in isolated mitochondria: proapoptotic Bax and Bak induce cytochrome c release and Δψ loss, leading to release of AIF, whereas antiapoptotic Bcl-2 and Bcl-xL prevent these changes (10–15). We and others have shown that Bax- and Bak-induced cytochrome c release and Δψ loss involve a polyprotein channel called the permeability transition (PT) pore (11, 12). This pore is considered to be formed at the site of contact between mitochondrial inner and outer membranes and to consist of the mitochondrial voltage-dependent anion channel (VDAC, also called porin), the adenine nucleotide translocator (ANT), cyclophilin D, and some other molecules (16, 17). Although some investigators have suggested that Bax did not induce Δψ loss (13–15), one of the features of PT pore opening in isolated mitochondria, their experiments were performed in the absence of Ca2+, and this cation is essential for PT pore opening. In the presence of a physiological concentration of Ca2+, Bax induces cytochrome c release in vitro via PT pore opening, which is characterized by Δψ loss, Ca2+ dependency, and cyclosporin A (CsA) sensitivity. We have shown recently that Bax binds directly to the VDAC and enhances channel activity to allow the passage of cytochrome c and also that VDAC opening is essential for Bax/Bak-induced Δψ loss (18). In addition, Bax was reported to bind to ANT and sensitize atractyloside-induced opening of the channel (12).
It has been shown recently that some of the BH3-only proteins, such as Bid and Bad, can transduce apoptotic signals to the mitochondria through posttranslational modifications (19). Phosphorylated Bad is localized in the cytoplasm through binding to 14-3-3, and dephosphorylation of Bad by calcineurin causes translocation to the mitochondria (20, 21). Dephosphorylated Bad binds to Bcl-xL and inhibits its antiapoptotic activity. It has been shown that Bid is also localized mainly in the cytoplasm and is cleaved by caspase-8 in tumor necrosis factor receptor and Fas signaling (22, 23). Truncated Bid (tBid) then translocates to the mitochondria and induces cytochrome c release. However, it has not been determined how the BH3-only proteins induce cytochrome c release. Here, we investigated the molecular mechanisms by which BH3-only proteins induced cytochrome c release. We found that, unlike Bax/Bak, BH3-only proteins induced cytochrome c release without opening the PT pore in isolated mitochondria and did not directly affect VDAC activity in liposomes.
Materials and Methods
Chemicals.
Anti-pigeon cytochrome c (7316A) and anti-human VDAC (31HL) mAbs were purchased from PharMingen and Calbiochem, respectively. Anti-human Bid polyclonal antibody (sc-6538) was from Santa Cruz Biotechnology. Lipofectamine was purchased from Life Technologies (Gaithersburg, MD). Dimethyl 3,3′-dithiobispropionimidate [2HCl] (DTBP) was obtained from Pierce. Bongkrekic acid (BK) was kindly provided by H. Terada and Y. Shinohara (Tokushima University, Japan). Diisopropylcarbodiimide/1-hydroxybenzotriazole-activated, fluorenylmethoxycarbonyl (Fmoc)-protected amino acids were obtained from Genzyme. Other chemicals were obtained from Wako.
Preparation of Isolated Mitochondria.
Livers of male Donryu rats were homogenized with a glass–Teflon Potter homogenizer. Mitochondria were isolated in 0.3 M mannitol/10 mM potassium-Hepes, pH 7.4/0.2 mM EDTA/0.1% fatty acid-free BSA, as described previously (24). The mitochondria were washed twice with and resuspended in the same medium without EDTA (MT-1 medium).
Protein Purification.
Human Bid, tBid, Bik, and mutant Bid (G94D95 to E94E95) were expressed as glutathione S-transferase (GST)-fusion proteins in Escherichia coli strain DH5α and purified on a glutathione-Sepharose column. Then these proteins were released from GST by cleavage with thrombin. Human Bax was expressed as a His-tagged protein in E. coli strain XL1-blue by using the Xpress System (Invitrogen), as described elsewhere (11). All purified proteins finally were suspended in the same control buffer composed of 20 mM Hepes-K+ (pH 7.4) and 1 mM DTT. Mock control proteins were prepared by using GST- and His-tagged proteins from empty vectors.
Rat liver mitochondrial VDAC was purified as described previously (18); it showed a single band on SDS-polyacrylamide gel.
Synthesis of BH3 Peptides.
Peptides, all corresponding to human proteins, were synthesized on a Model 396 Multiple Peptide Synthesizer (Advanced Chemtech) by using diisopropylcarbodiimide/1-hydroxybenzotriazole-activated, Fmoc-protected amino acids. The purity of each peptide was determined to be >90% by matrix-assisted laser desorption ionization–time of flight mass spectrometry.
Measurement of Mitochondrial Biochemical Parameters.
Isolated mitochondria (1 mg protein/ml) were incubated at 25°C in MT-1 medium plus 1 mM potassium phosphate and 4.2 mM succinate to energize the mitochondria (MT-2 medium), unless otherwise indicated. Δψ was assessed by measuring the Δψ-dependent uptake of rhodamine 123 using a spectrophotometer (Hitachi F-4500) as described elsewhere (24). For detection of cytochrome c release, mitochondria were spun and the supernatant was subjected to Western blot analysis by using anticytochrome c antibodies.
Reconstitution of VDAC in Liposomes.
Plain liposomes and VDAC liposomes were prepared at pH 5.2 (an acidic pH was required for efficient incorporation of recombinant Bcl-2 family proteins into the liposomes) as described previously (18). A sucrose import experiment was performed by assessing liposomal swelling. Liposomes (10 μl) were incubated in 1 ml of liposome medium together with recombinant proteins or oligopeptides for 3 min at 25°C. Then, sucrose was added to 50 mM, and liposomal swelling was assessed by the decrease of light scatter at a wavelength of 520 nm using a spectrophotometer (F-4500; Hitachi, Tokyo).
Immunoprecipitation and Western Blot Analysis.
Purified VDAC was incubated for 3 hr with either GST-Bid, GST-Bik, or GST-Bcl-xL. Then, these proteins were incubated with GSH-Sepharose for 3 hr. After brief centrifugation, the beads were washed and resuspended in sample buffer for SDS/PAGE. To detect binding between VDAC and Bcl-xL on VDAC liposomes, Bcl-xL (0.2 μg) was incubated for 3 hr with VDAC-containing liposomes (10 μl) with or without tBid (0.1 or 0.6 μg). Then, immunoprecipitation was performed as described previously (18).
Cell Culture and DNA Transfection.
293T cells were grown in DMEM supplemented with 10% FBS. Using lipofectamine, 293T cells were transfected for 24 hr with the expression plasmid (0.3 μg) for human Bax, human Bid, or empty vector together with 0.1 μg of the green fluorescence protein (GFP) expression construct pEGFP-N1 (CLONTECH). Then, cells were stained with 1 μM mitotracker red (MTR) and 1 μM biotin-conjugated annexin V plus 1 μM streptavidin-conjugated red 617 or 1 μM Hoechst 33342 and were analyzed with a flow cytometer (FACS Caliber; Becton Dickinson) and a fluorescent microscope (BX50; Olympus).
Results
Bid and Bik Induce Cytochrome c Release Without the Permeability Transition.
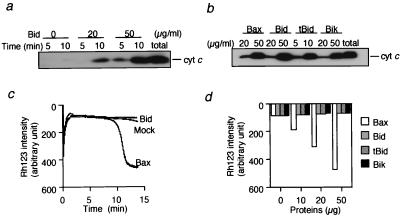
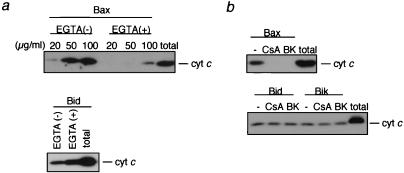
It has been reported that, like proapoptotic Bax and Bak, Bid induces cytochrome c release from the mitochondria (22, 23), which was confirmed in the present study (Fig. 1 a and b). Human recombinant Bid (rBid) induced cytochrome c release from isolated mitochondria in a dose- and time-dependent manner. N-terminal truncated rBid lacking amino acids 2–60 (rtBid) showed an approximately 5-fold stronger effect than rBid (Fig. 1b), consistent with previous findings (22). We also observed that Bik, another BH3-only protein, caused cytochrome c release to a similar extent as rBid and rBax (Fig. 1b). It has been reported that Bax- and Bak-induced cytochrome c release is accompanied by loss of mitochondrial Δψ when the mitochondria are incubated in the presence of Ca2+ (11, 12). As shown in Fig. 1 c and d, addition of rBax induced Δψ loss in a dose-dependent manner, confirming our previous observations (11), whereas rBid did not induce Δψ loss. Similar results also were obtained when rtBid and rBik were used (Fig. 1d). These results indicated that, unlike Bax and Bak, the BH3-only proteins induced cytochrome c release without Δψ loss. The lack of Δψ loss in association with BH3-only proteins indicated that these proteins did not induce the PT, unlike Bax/Bak. rBax-induced cytochrome c release was largely dependent on the PT, because cytochrome c release was almost completely inhibited by PT inhibitors, such as a Ca2+ chelator, EGTA (Fig. 2a), CsA, and BK (Fig. 2b), confirming previous observations (11). In contrast, Ca2+ chelation enhanced Bid-induced cytochrome c release (Fig. 2a), rather than inhibiting it, and CsA and BK also did not affect rBid- and rBik-induced cytochrome c release (Fig. 2b). These findings indicated that BH3-only proteins induced cytochrome c release without the PT.
Figure 1.
Induction of cytochrome c release by BH3-only proteins without affecting Δψ. (a and b) Induction of cytochrome c release by BH3-only proteins in isolated mitochondria. Mitochondria (1 mg/ml) were incubated with rBid, rBax, rtBid, or rBik at the indicated concentrations. At the indicated times (a) and at 10 min (b), samples were centrifuged and aliquots (20 μl) of the supernatants were subjected to Western blot analysis for cytochrome c (cyt c). “total” represents an equivalent aliquot of mitochondria. (c and d) No effect of BH3-only proteins on Δψ in isolated mitochondria. Mitochondria (1 mg/ml) were incubated with 50 μg/ml (c) or the indicated concentration (d) of rBax (open bar in d), rBid (shaded bar in d), rtBid (hatched bar in d), rBik (solid bar in d), or mock protein, and Δψ was measured by using rhodamine uptake over 15 min (c) or at 15 min (d).
Figure 2.
BH3-only proteins induce cytochrome c release without the PT. (a and b) Prevention of rBax-induced, but not Bid- and Bik-induced, cytochrome c release by Ca2+ chelation, CsA, and BK. Mitochondria (1 mg/ml) were incubated for 10 min with rBax (at the indicated concentrations in a and at 20 μg/ml in b) or with rBid (20 μg/ml) and rBik (20 μg/ml) in the presence or absence of 0.2 mM EGTA (a), 1 μM CsA (b), and 1 μM BK (b). Samples were centrifuged and aliquots (20 μl) of the supernatants were subjected to Western blot analysis for cytochrome c (cyt c). “total” represents an equivalent aliquot of mitochondria.
Involvement of Distinct Mechanisms in Bax- and BH3-Only Protein-Induced Cytochrome c Release.
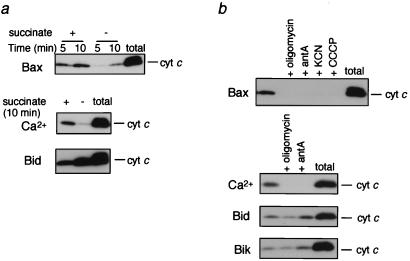
Bax-induced apoptosis and cytochrome c release in isolated mitochondria have been reported to be inhibited by oligomycin, an F0F1ATPase inhibitor (25). As shown in Fig. 3a, Bax-induced cytochrome c release also was markedly inhibited in the absence of succinate (a respiratory substrate). In addition to oligomycin, inhibitors of respiration, such as antimycin A and KCN (which target complex III and complex IV, respectively) or carbonylcyanide-m-chlorophenylhydrazone (CCCP, a respiratory uncoupler), caused almost complete inhibition of Bax-induced cytochrome c release (Fig. 3b), indicating that this cytochrome c release was largely dependent on efficient respiration. Similar results also were observed when mitochondria were treated with Ca2+ (Fig. 3). In contrast, Bid- and Bik-induced cytochrome c release was enhanced in the absence of succinate or by addition of antimycin A and was only slightly inhibited by oligomycin (Fig. 3). These results indicated that, unlike Bax, BH3-only proteins induced cytochrome c release by a mechanism that was independent of mitochondrial respiration.
Figure 3.
Prevention of Bax- and Ca2+-induced, but not Bid-induced, cytochrome c release by inhibition of respiration. Mitochondria (1 mg/ml) were incubated with rBax (20 μg/ml), Ca2+ (50 μM), or rBid (20 μg/ml) in the presence or absence of 5 mM succinate (a) or were incubated with or without 10 μM oligomycin, 10 μM antimycin A (antA), 1 mM KCN, or 1 mM CCCP (b). At the indicated times (a) and at 10 min (b), samples were centrifuged and aliquots (20 μl) of the supernatants were analyzed by Western blotting by using anticytochrome c antibody.
BH3 Domain of BH3-Only Proteins Induces Δψ Loss and Cytochrome c Release.
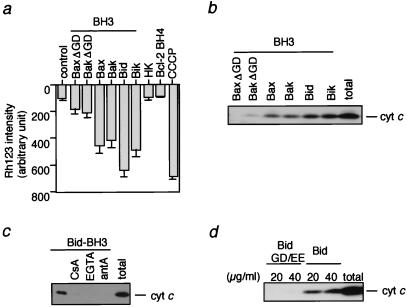
It has been shown that the BH3 domain of Bax/Bak and Bid/Bik is essential for their proapoptotic activity as well as for cytochrome c release (1–3). The difference in cytochrome c release induced by Bax/Bak and BH3-only proteins might depend on distinct functions of their respective BH3 domains. To test this possibility, we compared the effect of oligopeptides corresponding to the BH3 region of Bax/Bak and Bid/Bik. As shown in Fig. 4 a and b, both Δψ loss and cytochrome c release were induced to a similar extent by all BH3 peptides derived from human Bid, human Bik, human Bax, and human Bak. Furthermore, cytochrome c release and Δψ loss induced by the BH3 peptide of Bid were almost completely inhibited by CsA, Ca2+ chelation, and antimycin A (Fig. 4c and data not shown), also consistent with the results of BH3 peptide of Bax (11). Mutant BH3 oligopeptides (BaxΔGD and BakΔGD) were unable to induce cytochrome c release and Δψ loss (Fig. 4 a and b), confirming an essential role of the BH3 domain in the proapoptotic activity of Bax/Bak. The absence of Δψ loss and cytochrome c release by an irrelevant peptide derived from hexokinase and BH4 peptide of Bcl-2 excluded the possibility of nonspecific peptide toxicity (Fig. 4a and data not shown). As shown in Fig. 4d, a BH3 mutant of Bid (BidGD/EE: G94D95 to EE) did not induce cytochrome c release, also confirming an essential role of the BH3 domain of Bid in cytochrome c release. These results indicated that there were no substantial differences between the activity of the BH3 domain of BH3-only proteins and that of Bax or Bak, suggesting that region(s) other than the BH3 domain of Bid/Bik contributed to the difference between Bax/Bak- and Bid/Bik-induced cytochrome c release.
Figure 4.
Role of the BH3 domain in Bid-induced cytochrome c release. (a and b) Induction of Δψ loss and cytochrome c release by BH3 peptides. Mitochondria (1 mg/ml) were incubated in the presence of the following peptides (10 μM), and Δψ (a) and cytochrome c release (b) were measured at 10 min: Bax (amino acid residues 55–74), Bak (amino acid residues 73–87), Bid (amino acid residues 82–101), Bik (amino acid residues 53–72), BaxΔGD (Bax peptide lacking G67D68), and BakΔGD (Bak peptide lacking G82D83). Peptides derived from hexokinase (HK; amino acid residues 2–21) and Bcl-2 BH4 (amino acid residues 10–30) also were added as controls. Complete loss of Δψ was demonstrated by incubation of mitochondria with 1 mM CCCP for 10 min. (c) Prevention of BH3 peptide-induced cytochrome c release by CsA, Ca2+ chelation, and antimycin A. Mitochondria (1 mg/ml) were incubated for 10 min with Bid BH3 peptide (10 μM) in the presence of 1 μM CsA, 0.2 mM EGTA, and 10 μM antimycin A (antA). (d) Requirement of the BH3 domain for Bid-induced cytochrome c release. Mitochondria (1 mg/ml) were incubated with rBid and mutant Bid GD/EE (G94D95 to E94E95) at the indicated concentrations for 10 min, and cytochrome c release was measured as described in Materials and Methods. “total” in b–d represents an equivalent aliquot of mitochondria.
BH3-Only Proteins Do Not Influence VDAC Activity.
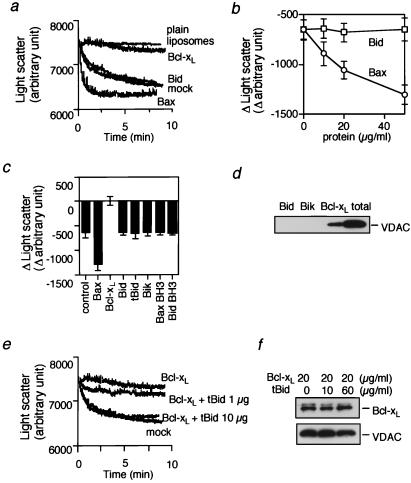
We recently have shown that Bcl-xL and Bax bind directly to the VDAC and antagonistically modulate the activity of this channel, thus regulating apoptotic cytochrome c release and Δψ loss (18). Therefore, we examined the effect of the BH3-only proteins on VDAC activity. We measured VDAC activity by assessing sucrose uptake into VDAC-containing liposomes, which caused swelling of the liposomes that was monitored by the decrease of light scatter by using a spectrophotometer (Fig. 5a). Sucrose uptake-dependent liposomal swelling was confirmed by microscopic observations and flow cytometric analysis (data not shown) and explained by the rapid influx of sucrose and water through large VDAC pores, which overwhelmed the osmosis-dependent efflux of water. VDAC liposomes developed swelling in the presence of sucrose, whereas neither plain liposomes nor heat-denatured VDAC liposomes showed swelling (Fig. 5a and data not shown), indicating that sucrose uptake was mediated by the VDAC. As shown in Fig. 5 a and b, addition of rBcl-xL to VDAC liposomes inhibited VDAC-mediated sucrose uptake and rBax enhanced it in a dose-dependent manner, confirming our previous observations (18). In contrast, rBid did not affect VDAC activity (Fig. 5 a and b). Similar results also were obtained with rBik, rtBid, and BH3 oligopeptides derived from Bax and Bid (Fig. 5c). Consistently, unlike rBax and rBak, it was found that rBid and rBik did not allow cytochrome c to pass through the VDAC in VDAC liposomes (ref. 18 and data not shown). Furthermore, rBid and rBik did not bind directly to the VDAC (Fig. 5d), whereas Bcl-xL bound to VDAC, confirming our previous observation (18). These results indicated that, unlike Bax and Bak, BH3-only proteins and BH3 oligopeptides did not directly influence VDAC activity.
Figure 5.
No direct effect of Bid on VDAC activity. (a and b) Inability of Bid to directly modulate VDAC activity. Plain liposomes and VDAC liposomes were incubated with 50 mM sucrose together with rBcl-xL, rBax, or rBid at 20 μg/ml (a) or at indicated concentrations (b) and with an equivalent amount of mock protein. In a, liposome swelling was assessed continuously for 10 min by the decrease of light scatter by using a spectrophotometer at a wavelength of 520 nm. In b, the changes of light scatter at 10 min from the initial value (time 0) are shown. (c) Inability of BH3-only proteins and BH3 peptides to modulate VDAC activity. VDAC liposomes were incubated with 50 mM sucrose with or without rBax, rBcl-xL, rBid, rtBid, or rBik at 50 μg/ml or with or without Bax BH3 peptide and Bid BH3 peptide at 10 μM, and the differences of light scatter at 10 min from the initial value (time 0) are shown. (d) No direct interaction of Bid and Bik with VDAC. Purified rat liver VDAC (0.2 μg) was incubated with GST-Bid, GST-Bik, or GST-Bcl-xL fusion protein (0.2 μg each), followed by incubation with GSH-Sepharose as described in Materials and Methods. After washing three times, the bound VDAC was analyzed by Western blotting. “total” represents the amount of VDAC used for the experiment. (e) Effect of tBid on Bcl-xL activity to inhibit VDAC activity. VDAC liposomes were incubated with 50 mM sucrose together with mock protein or Bcl-xL (20 μg/ml) in the presence or absence of tBid at the indicated concentrations, and the changes of light scatter were monitored continuously. (f) Effect of tBid on interaction between Bcl-xL and VDAC in VDAC liposomes. VDAC liposomes (20 μl) were incubated with Bcl-xL (20 μg/ml) in the presence of tBid at the indicated concentrations. These samples were subjected to immunoprecipitation with an anti-VDAC antibody, and the immune complexes were analyzed by Western blotting by using anti-VDAC and anti-Bcl-xL antibodies as described in Materials and Methods.
Because Bid has been shown to physically interact with Bcl-xL, it is conceivable that Bid exerts its proapoptotic activity by antagonizing antiapoptotic Bcl-2 family members. As shown in Fig. 5 e and f, the activity of Bcl-xL to inhibit VDAC-dependent sucrose uptake was reduced by the addition of tBid in a dose-dependent manner, but tBid did not affect the physical interaction between VDAC and Bcl-xL. These results suggested that Bid might exert the proapoptotic activity by inhibiting the activity of antiapoptotic Bcl-2 family members.
Bid Induces Apoptosis Without Reducing Δψ.
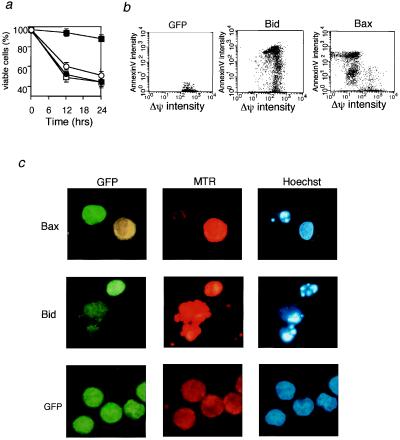
Because Bax has been shown to induce Δψ loss in cells (26) as well as in isolated mitochondria (11, 12), the results described above raised the possibility that Bid might induce apoptosis without affecting mitochondrial Δψ. 293T cells were transiently transfected with an expression construct for Bid or Bax together with an expression construct for GFP to identify cells transfected with the DNAs. After staining with MTR to monitor mitochondrial Δψ and with annexin V and Hoechst 33342 to detect apoptosis, cells were analyzed by using a flow cytometer and a fluorescent microscope. As can be seen in Fig. 6 a and b, Δψ loss preceded Bax-induced apoptosis, as shown by the presence of annexin V+-Δψlow cells and the absence of annexin V+-Δψhigh cells, whereas Δψ loss did not accompany Bid-induced apoptosis. Furthermore, as displayed in Fig. 6c, Bax-transfected cells (GFP-positive cells) showed apoptotic chromatin condensation (detected by Hoechst staining) and a reduced Δψ (weak MTR staining). In contrast, Bid-transfected cells (GFP-positive cells) showed apoptotic chromatin condensation while maintaining Δψ (stronger MTR staining) like intact cells (GFP-transfected cells). These results indicated that Bax-induced apoptosis, but not Bid-induced apoptosis, was accompanied by mitochondrial Δψ loss, consistent with the results obtained by using isolated mitochondria.
Figure 6.
Decrease of mitochondrial Δψ by transfection of bax but not bid. (a) Effect of transient expression of Bax and Bid on mitochondrial Δψ and apoptosis. 293T cells were transfected with an expression construct (0.3 μg) for Bax (open symbols) and Bid (solid symbols), together with 0.1 μg of the GFP expression construct (for detection of DNA-transfected cells). At the indicated times, transfected cells were stained with MTR for measuring Δψ and with biotin-conjugated annexin V and streptavidin-red 617 for detecting apoptosis. Then, apoptosis (circles) and Δψ (squares) were measured by using a flow cytometer. The fractions of apoptotic cells and low Δψ cells were determined as the percentage of annexin V-positive, GFP-positive cells and MTR-negative, GFP-positive cells, respectively, among all GFP-positive cells. Data are shown as the mean ± SD for three independent experiments. (b) Representative flow cytometric profile of Bid- and Bax-overexpressing cells. The same experiments as in a were performed. Empty vector (0.3 μg) together with 0.1 μg of the GFP expression construct also was transfected as a control. After 24 hr, GFP-positive cells were gated and apoptosis (annexin V) and Δψ were analyzed by using a flow cytometer. (c) Representative morphology of bax- and bid-transfected cells. 293T cells were transfected with an expression construct (0.3 μg) for Bax, Bid, or empty vector, together with 0.1 μg of the GFP expression construct. After 24 hr, cells were stained with MTR to monitor Δψ and with Hoechst 33342 to assess nuclear morphology and were observed by using a fluorescent microscope. GFP-positive cells were stained green, and cells maintaining their Δψ were strongly stained orange (MTR).
Discussion
It has been reported that rBax/rBak induces cytochrome c release as well as the PT (Δψ loss) in isolated mitochondria (11, 12). In contrast, we showed here that rBid and rBik induced cytochrome c release but not the PT in isolated mitochondria, based on (i) the absence of Δψ loss and (ii) the lack of inhibition of cytochrome c release by PT inhibitors such as CsA, BK, and a Ca2+ chelator (Figs. 1 and 2). Although some investigators have reported that Bax induces cytochrome c release via a PT (Δψ loss)-independent mechanism (13–15), this discrepancy was due to differences in the mitochondrial assay medium, that is, the presence or absence of Ca2+ ion, which is essential for the PT. Because CsA has been shown to significantly inhibit Bax-induced apoptosis in several cell lines (ref. 27 and our unpublished observations), induction of the PT or its underlying mechanism seems to be crucial for Bax-induced apoptosis in vivo, which is consistent with our findings with isolated mitochondria.
Recently, we and others showed that Bnip3L, E1B 19-kDa-interacting protein 3-like protein (also called Nix), possesses BH3 domain but not BH1, 2, and 4 and has proapoptotic activity (28–30). Unlike Bid and Bik, this protein induced both cytochrome c release and Δψ loss in isolated mitochondria (29). However, Bnip3L seems to be significantly different from the BH3-only proteins such as Bid/Bik in that, unlike Bid and Bik, deletion of BH3 domain from Bnip3L only partially abrogated its proapoptotic ability (28, 29), suggesting that Bnip3L has an additional proapoptotic function, which might be responsible for Δψ loss.
Bax induces cytochrome c release and the PT by directly interacting with the VDAC, an important component of the PT pore, and enhancing its channel activity (18), whereas, consistent with the observations that BH3-only proteins induced cytochrome c release without the PT, Bid/Bik showed neither structural nor functional interaction with the VDAC (Fig. 5). These results raised the possibility that Bid and Bik target molecule(s) other than the PT pore component or act alone to induce cytochrome c release but not Δψ loss. Alternatively, because Bid/Bik is able to interact with other Bcl-2 family members localized on the mitochondria (31–33) (such as Bak, Bax, and Bcl-xL), all of which directly regulate VDAC activity (18), Bid/Bik might function as a ligand for other Bcl-2 family members, as proposed previously (31), which regulates these members and enhances VDAC activity while somehow inhibiting Δψ loss. Indeed, we showed here that tBid suppressed the activity of Bcl-xL to inhibit VDAC activity. Thus, the heterodimerization of Bid to Bcl-xL probably underlies at least partly the proapoptotic activity of Bid.
Unlike Bid and Bik proteins, BH3 oligopeptides from Bid and Bik induced both cytochrome c release and Δψ loss like Bax and Bak in a PT-dependent manner, suggesting that a region of Bid/Bik other than the BH3 domain might inhibit BH3 domain-induced PT, or, alternatively, it might have affinity to molecules other than PT pore components. In any case, the BH3 domains of Bid and Bik are essential for their proapoptotic activity and for cytochrome c release (Fig. 4), as is the case for Bax/Bak. Because BaxΔBH3 still is able to bind to the VDAC but does not enhance its activity (18), this indicates binding by Bax to the VDAC at a site other than BH3 and subsequent opening of the VDAC probably by using the BH3 region. Similarly, BH3-only proteins might bind to other channels and modify their function by using the BH3 domain. Because it is unlikely that BH3 peptides act alone, these peptides probably function through the VDAC by activating Bax/Bak or through binding to other channel(s), as described above.
F0F1-ATPase is implicated in Bax-induced apoptosis and cytochrome c release because (i) Bax is lethal for wild-type but not mutant yeast lacking ATP4 (an F0F1-ATPase component) and (ii) oligomycin makes mammalian cells resistant to Bax-induced apoptosis and cytochrome c release (25). Here, we showed that Bax/Bak- and Ca2+-induced cytochrome c release but not Bid/Bik-induced cytochrome c release was suppressed by oligomycin and by inhibition of the respiratory chain with succinate depletion, KCN, and antimycin A. Bax/Bak-induced cytochrome c release also was inhibited by an uncoupler, CCCP. Although it is still unclear how these drugs influence Bax- and Ca2+-induced cytochrome c release, these results are consistent with previous findings that reoxygenation but not hypoxia induces the PT (34), which is prevented by inhibitors of respiration.
We showed here that Δψ loss was induced by transfection with bax but not bid. There have been conflicting reports on the role of Δψ in apoptosis: some authors have found that Δψ loss precedes apoptosis (35, 36), and others have suggested that apoptosis is not accompanied by Δψ loss (5, 6). Our findings might help to explain this discrepancy. For example, Fas-induced apoptosis involving the mitochondria in the signal transduction often is not associated with Δψ loss (37, 38), and this might be due to caspase-8-dependent activation of Bid (22, 23, 39) that does not induce Δψ loss, as described here. In contrast, chemotherapy-induced apoptosis often is accompanied by Δψ loss (33, 34), which might be due to the activation of Bax/Bak or their relatives in the Bcl-2 family. Thus, different proapoptotic Bcl-2 members might be involved in apoptosis depending on the cell type and death stimulus, thereby variously resulting in reduction or maintenance of Δψ during apoptosis.
Acknowledgments
We are grateful to Drs. J. Yuan and G. Chinnadurai for providing bid and bik cDNA, respectively, and Drs. K. Uegaki and N. Yumoto (Osaka National Research Institute) for providing some oligopeptides. This study was supported in part by grants for Scientific Research on Priority Areas, for Center of Excellence Research, and for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Abbreviations
- PT
permeability transition
- VDAC
voltage-dependent anion channel
- CsA
cyclosporine A
- Δψ
mitochondrial membrane potential
- GFP
green fluorescence protein
- MTR
mitotracker red
- BK
bongkrekic acid
- rBid
human recombinant Bid
- rtBid
N-terminal truncated rBid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Adams J M, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Tsujimoto Y. Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 3.Green D R, Reed J C. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 6.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 7.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Nature (London) 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 8.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 9.Thornberry N A, Lazebnil Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 10.Jürgensmeier J M, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed J C. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narita M, Shimizu S, Ito T, Chittenden T, Lutz R J, Matsuda H, Tsujimoto Y. Proc Natl Acad Sci USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzo I, Brenner C, Zamzami N, Jürgensmeier J M, Susin S A, Vieira H L, Prevost M C, Xie Z, Matsuyama S, Reed J C, et al. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 13.Eskes R, Antonsson B, Osen-Sand A, Montessuit S C, Sadoul R, Mazzei G, Nichols A, Martinou J C. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finucane D M, Bossy-Wetzel E, Waterhouse N J, Cotter T G, Green D R. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 15.Priault M, Chaudhuri B, Clow A, Camougrand N, Manon S. Eur J Biochem. 1999;260:684–691. doi: 10.1046/j.1432-1327.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 16.Bernardi P, Broekemeier K M, Pfeiffer D R. J Bioenerg Biomembr. 1994;26:509–517. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 17.Zoratti M, Szabó I. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu S, Narita M, Tsujimoto Y. Nature (London) 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 19.Korsmeyer S J. Cancer Res. 1999;59:1693S–1700S. [PubMed] [Google Scholar]
- 20.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang H G, Pathan N, Ethell I M, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke T F, Reed J C. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Zhu H, Xu C J, Yuan J. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 23.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Proc Natl Acad Sci USA. 1998;95:1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuyama S, Xu Q, Velours J, Reed J C. Mol Cell. 1998;1:327–336. doi: 10.1016/s1097-2765(00)80033-7. [DOI] [PubMed] [Google Scholar]
- 26.Xiang J, Chao D T, Korsmeyer S J. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastorino J G, Chen S T, Tafani M, Snyder J W, Farber J L. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Cizeau J, Vande Velde C, Park J H, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A. J Biol Chem. 1999;274:7–10. doi: 10.1074/jbc.274.1.7. [DOI] [PubMed] [Google Scholar]
- 29.Imazu T, Shimizu S, Tagami S, Matsushima M, Nakamura Y, Miki T, Okuyama A, Tsujimoto Y. Oncogene. 1999;18:4523–4529. doi: 10.1038/sj.onc.1202722. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda M, D'Sa-Eipper C, Gong X L, Chinnadurai G. Oncogene. 1998;17:2525–2530. doi: 10.1038/sj.onc.1202467. [DOI] [PubMed] [Google Scholar]
- 31.Wang K, Yin X M, Chao D T, Milliman C L, Korsmeyer S J. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 32.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J C. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd J M, Gallo G J, Elangovan B, Houghton A B, Malstrom S, Avery B J, Ebb R G, Subramanian T, Chittenden T, Lutz R J, et al. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- 34.Shimizu S, Kamiike W, Hatanaka N, Nishimura M, Miyata M, Inoue T, Yoshida Y, Tagawa K, Matsuda H. Transplantation. 1994;57:144–148. doi: 10.1097/00007890-199401000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Oncogene. 1996;13:21–29. [PubMed] [Google Scholar]
- 37.Banki K, Hutter E, Gonchoroff N J, Perl A. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 38.Weis M, Schlegel J, Kass G E, Holmstrom T H, Peters I, Eriksson J, Orrenius S, Chow S C. Exp Cell Res. 1995;219:699–708. doi: 10.1006/excr.1995.1281. [DOI] [PubMed] [Google Scholar]
- 39.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]