Abstract
The yeast Saccharomyces cerevisiae expressing a cDNA library prepared from Stylosanthes hamata was screened for enhanced Mn2+ tolerance. From this screen, we identified four related cDNAs that encode membrane-bound proteins of the cation diffusion facilitator (CDF) family. One of these cDNAs (ShMTP1) was investigated in detail and found to confer Mn2+ tolerance to yeast by internal sequestration rather than by efflux of Mn2+. Expression of ShMTP1 in a range of yeast mutants suggested that it functions as a proton:Mn2+ antiporter on the membrane of an internal organelle. Similarly, when expressed in Arabidopsis, ShMTP1 conferred Mn2+ tolerance through internal sequestration. The ShMTP1 protein fused to green fluorescent protein was localized to the tonoplast of Arabidopsis cells but appeared to localize to the endoplasmic reticulum of yeast. We suggest that the ShMTP1 proteins are members of the CDF family involved in conferring Mn2+ tolerance and that at least one of these proteins (ShMTP1) confers tolerance by sequestering Mn2+ into internal organelles.
INTRODUCTION
Proteins belonging to the cation diffusion facilitator (CDF) family (also known as the cation efflux family) have been implicated in the metal tolerance mechanisms of a range of organisms. Indirect evidence suggests that these proteins are transporters that either sequester metal ions within cells or export metal ions out of cells (Paulsen and Saier, 1997). The CDF proteins described to date confer Zn2+, Cd2+, Co2+, or Ni2+ tolerance to a range of organisms, and many are located on internal membranes. In at least one instance, a protein of the CDF family was shown to be involved in Zn2+ uptake rather than Zn2+ efflux across the plasma membrane, indicating diverse functional roles for this class of transporters (Cragg et al., 2002). The mechanism of transport and the driving force that allows the metal ions to be transported are not well understood for most of the CDF proteins.
Plants possess a range of genes that encode CDF proteins, and there is evidence that some of these are involved in metal tolerance. For example, the ZAT gene of Arabidopsis confers increased Zn2+ tolerance when overexpressed in plants (van der Zaal et al., 1999). The increased tolerance is thought to be attributable to internal sequestration of Zn2+; however, the subcellular location of the protein and the site where Zn2+ is sequestered are unknown. Bloß et al. (2002) expressed ZAT in Escherichia coli and studied the purified protein in reconstituted proteoliposomes. The protein transported Zn2+ into proteoliposomes by a mechanism that relied on the Zn2+ gradient across the membrane and not on a proton gradient. Persans et al. (2001) isolated genes (TgMTPs) that encode CDF proteins from the nickel-hyperaccumulating species Thlaspi goesingense, which conferred metal tolerance to Saccharomyces cerevisiae mutants defective in COT1 and ZRC1. Both COT1 and ZRC1 are yeast proteins of the CDF family that confer Zn2+ and Co2+ tolerance and are located on the vacuolar membrane (Li and Kaplan, 1998). Recently, MacDiarmid et al. (2002) provided evidence that ZRC1 acts as a proton antiporter to transport Zn2+ into the vacuole. Persans et al. (2001) also suggested that the TgMTP1 proteins are involved in transporting metals to the vacuole, although the location of the proteins in vivo and their transport activities have yet to be demonstrated in either yeast or plants.
Proteins of the CDF family from diverse sources have the following features in common: (1) they share an N-terminal signature sequence that appears to be specific to the family; (2) the proteins possess six transmembrane-spanning regions; (3) they share a cation efflux domain; and (4) most of the eukaryotic members possess an intracellular His-rich domain that is absent from the prokaryotic members (Paulsen and Saier, 1997). Initial analysis of the Arabidopsis genome identified eight genes that encode proteins belonging to the CDF family (Mäser et al., 2001). Four of the putative proteins encoded by these genes possess all of the features described above that are common to the CDF family, whereas the other, more distantly related proteins (previously named AtMTPc1 to AtMTPc4 for metal tolerance protein) possess only a subset of these features (Mäser et al., 2001). The AtMTPc proteins have four to five predicted transmembrane domains; three of them possess only part of the N-terminal signal sequence, and all lack the His-rich domain.
Stylosanthes hamata is a tropical legume tolerant of acid soils in which high concentrations of soil Mn2+ can occur and represent a potential source of genes that confer Mn2+ tolerance (de Carvalho et al., 1980). Here, we describe the isolation of cDNAs from Stylosanthes that encode proteins of the CDF family with features similar to those of the previously named AtMTPc3 of Arabidopsis (Mäser et al., 2001). One of these cDNAs was characterized in detail and shown to confer Mn2+ tolerance to yeast and plants by a mechanism that is likely to involve the sequestration of Mn2+ into internal organelles.
RESULTS
Isolation of Stylosanthes cDNAs That Confer Mn Tolerance to Yeast
A yeast expression library was screened on agar medium that contained Mn2+ at a concentration that was toxic to wild-type Saccharomyces. From this screen, colonies were selected that grew vigorously on the Mn2+-toxic agar. To confirm that expression of the plant cDNAs was responsible for the Mn2+-tolerance phenotype, the plasmids were isolated and transformed into the wild-type parental strain and rescreened on Mn2+-toxic agar. Sequencing showed that the cDNAs encoded four related proteins with features similar to those of the CDF family of transporters.
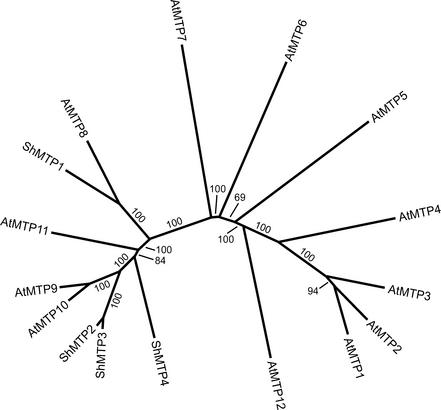
A search of the databases showed a strong similarity of the Stylosanthes proteins to four proteins encoded by putative genes in the Arabidopsis genome (Figure 1). One of the Arabidopsis proteins, previously named AtMTPc3 (Mäser et al., 2001), was identified by homology as a member of the CDF family of transporters. The other three Arabidopsis proteins were not recognized previously as CDF proteins, and it is now apparent that they, along with AtMTPc3, form a distinct subgroup within the Arabidopsis CDF family (Figure 2). To simplify the nomenclature and to avoid the potential difficulties of defining subgroups by the inclusion of lowercase letters, the Arabidopsis genes that encode the CDF proteins have been renamed AtMTP1 to AtMTP12 (David Salt, personal communication). In keeping with this nomenclature, we named the Stylosanthes genes identified in the Mn2+-tolerance screen ShMTP1 to ShMTP4. As a result of their phylogenetic relationship to AtMTP1, three other proteins from the Arabidopsis CDF family, AtMTP2, AtMTP3, and AtMTP4, are thought to be Zn2+ transporters (Mäser et al., 2001) (Figure 2); AtMTP1 also is known as ZAT.
Figure 1.
Analysis of the MTP Family of Proteins from Stylosanthes and Related Proteins from Arabidopsis.
CLUSTAL W alignment of the MTP proteins from Stylosanthes and Arabidopsis. Identical amino acids are indicated with dark shading, and similar amino acids are indicated with light shading. The membrane-spanning domains for ShMTP1 identified by TMHMM (version 2.0; http://www.cbs.dtu.dk/services/TMHMM/) are shown as lines above the sequence. The Arg residue at position 123, which when mutated to Ile abolishes the ability of ShMTP1 to confer Mn2+ tolerance, is circled. The partially conserved N-terminal signature sequence for CDF proteins identified by Paulsen and Saier (1997) is boxed. The fully conserved Ser and Asp residues within this sequence are indicated by asterisks. The Arabidopsis genes are denoted by the names provided by the PlantsT World Wide Web site (http://plantst.sdsc.edu/). AtMTP8 originally was named AtMTPc3 (Mäser et al., 2001).
Figure 2.
Phylogenetic Relationships of the CDF Proteins from Stylosanthes and Arabidopsis.
The unrooted tree was drawn using PHYLIP (Felsenstein, 1989) after the sequences were aligned with CLUSTAL W (Thompson et al., 1994). The branch values indicate the number of times (%) that each branch topology was found during bootstrap analysis.
The proteins AtMTP8 to AtMTP11 along with ShMTP1 to ShMTP4, although related to this clade, are clustered as a major group, and evidence presented here suggests that they are CDF proteins involved primarily in Mn2+ transport. The translation products of rice ESTs and Caenorhabditis elegans sequences also showed strong similarity to the ShMTP family of proteins, indicating that the distribution of these types of proteins is not restricted to plants. The molecular masses of the ShMTP proteins are predicted to be 46.7 kD (ShMTP1; 416 amino acid residues), 46.4 kD (ShMTP2; 407 amino acid residues), 47.0 kD (ShMTP3; 414 amino acid residues), and 47.4 kD (ShMTP4; 416 amino acid residues). The amino acid sequences of the ShMTP proteins did not show significant similarity to other proteins that are known to transport Mn2+, such as CAX2 (Hirschi et al., 2000), members of the NRAMP family (Mäser et al., 2001), Ca2+/Mn2+-ATPases (Lapinskas et al., 1995), and ABC-type ATPases (Bartsevich and Pakrasi, 1996).
Analysis of the Stylosanthes sequences showed that, like the related Arabidopsis proteins (AtMTP8 to AtMTP11), they lack the complete N-terminal signature sequence for the CDF family and lack a His-rich region that is found commonly in eukaryotic members of the CDF family. Furthermore, transmembrane prediction programs identified models for the ShMTP family consisting of either four (http://www.cbs.dtu.dk/services/TMHMM/) or five (http://www.ch.embnet.org/software/TMPRED_form.html) membrane-spanning regions. Although the ShMTP and related Arabidopsis proteins lack the complete N-terminal signature sequence for the CDF family, all possess Ser and Asp residues (Figure 1) that are found to be fully conserved in other members of the CDF family (Paulsen and Saier 1997). All of these sequences possess a cation efflux domain, which is indicative of the CDF family as identified by the Pfam protein domain database (http://pfam.wustl.edu). Although the ShMTP1 protein lacks the complete N-terminal sequence suggested by Paulsen and Saier (1997) to be a signature sequence for the CDF class of proteins, we show here that the expression of ShMTP1 in yeast and Arabidopsis confers phenotypes consistent with a role in Mn2+ transport (see below). This finding indicates that the N-terminal signature sequence in itself is insufficient to identify all members of the CDF family, whereas the cation efflux domain is a more reliable indicator for this class of proteins.
Expression of ShMTP1 in Yeast Confers Mn2+ Tolerance
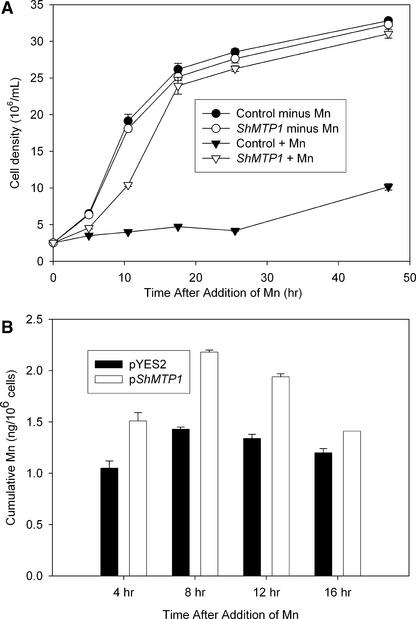
Expression of ShMTP1 in yeast conferred a high level of Mn2+ tolerance on both solid (data not shown) and liquid media (Figure 3A). At Mn2+ concentrations of <7.5 mM, no difference in growth rate was observed between cells expressing ShMTP1 and control cells for the INVSc2 strain (data not shown). Analysis of cells growing in liquid medium with 5 mM Mn2+, a concentration that did not restrict cell growth, showed that the yeast expressing ShMTP1 accumulated ∼20 to 50% more Mn2+ than control cells at each growth stage sampled over 16 h (Figure 3B). Because growth rates over the 16 h were similar for both genotypes, differences in Mn2+ accumulation could not be explained either by differences in the dilution of cellular Mn2+ caused by differing growth rates or by differences in cell viability. Similarly, when cells were exposed to 40 mM Mn2+, a concentration that eventually was toxic to control cells, ShMTP-expressing cells accumulated more Mn2+ than control cells over the initial 8 h of exposure (data not shown). These patterns of accumulation are consistent with an internal sequestration of Mn2+ rather than with an efflux of Mn2+ to the external medium. We would have expected less Mn2+ to be accumulated in the Mn2+-tolerant yeast compared with the control had the mechanism relied on Mn2+ efflux from cells.
Figure 3.
Effect of ShMTP1 Expression on Mn2+ Tolerance and Mn2+ Accumulation of Saccharomyces Strain INVSc2.
(A) Liquid medium inoculated with Saccharomyces to an initial density of 2.5 × 106 cells per mL was subsampled periodically to monitor growth at basal Mn2+ concentration (circles) or in the presence of 40 mM Mn2+ (inverted triangles). Closed symbols represent the growth of yeast transformed with the empty vector, and empty symbols represent the growth of yeast expressing ShMTP1.
(B) Mn accumulation by Saccharomyces transformed with ShMTP1 (open columns) or empty vector (control; closed columns). Mn2+ (5 mM) was added to yeast cultures at an initial density of 2.5 × 106 cells per mL, and subsamples were collected at the time intervals shown. The growth of both genotypes was unrestricted by 5 mM Mn2+ and was similar to the growth of cells in basal Mn (as shown in [A]) over 16 h. The yeast depleted Mn2+ in the medium by <1% during the experiment. Data shown are means and standard errors (n = 3).
Expression of ShMTP1 in yeast specifically conferred tolerance to Mn2+, and no increased tolerance or sensitivity was observed to a range of other metals (Cu2+, Ni2+, Co2+, Hg2+, Zn2+, Al3+, and Cd2+ [data not shown]). The ionic radius of Mn2+ is similar to that of Ca2+, and the two ions can substitute for one another in many biological functions (Loukin and Kung, 1995). For instance, the Arabidopsis CAX1 protein is a specific Ca2+ transporter, but the related protein CAX2 is able to transport other metal ions, including Mn2+, and its overexpression in plants confers a degree of Mn2+ tolerance (Hirschi et al., 2000). Because CAX1 and CAX2 were isolated initially by their ability to complement a Ca2+-sensitive yeast mutant (Hirschi et al., 1996), the ability of ShMTP1 to complement a similar mutant also was assessed. However, ShMTP1 was unable to complement the K667 yeast mutant with defects in Ca2+ transporters at the vacuole (Cunningham and Fink, 1996), suggesting that it is unable to sequester Ca2+ within internal organelles or to export Ca2+ out of yeast cells (data not shown).
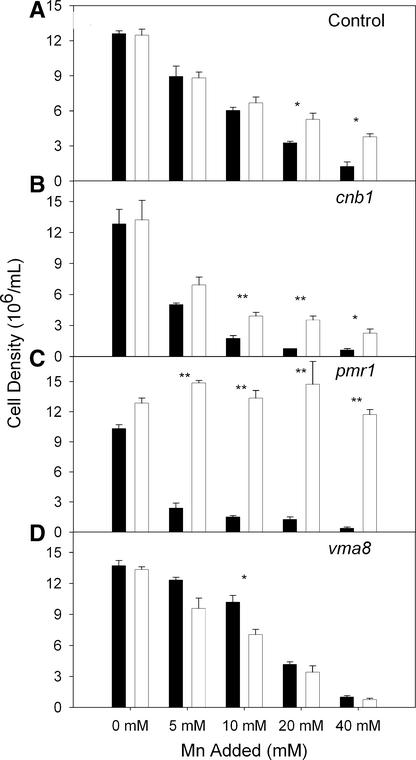
To obtain clues regarding the mechanism of Mn2+ tolerance conferred by the ShMTP1 protein, ShMTP1 was expressed in a range of previously described Mn2+-sensitive yeast mutants (Figure 4). The cnb1 mutant is defective in Ca homeostasis as a result of inactive calcineurin, and its sensitivity to Mn2+ is thought to be the result of CNB1 also being involved in Mn2+ homeostasis (Farcasanu et al., 1995). The increased sensitivity of pmr1 mutants to Mn2+ has been attributed to the absence of the Ca2+/Mn2+-ATPase activity located at the Golgi complex, which normally acts to reduce cytosolic Mn2+ concentrations, with subsequent export of Mn2+ to the external medium by exocytosis (Lapinskas et al., 1995). The VMA8 gene encodes the D subunit of the catalytic domain of the V-type H+-ATPase, and mutations in this gene prevent yeast from effectively acidifying their vacuoles (Xu and Forgac, 2000). Yeast mutants that are unable to acidify their vacuoles have increased sensitivity to a range of metals, including Mn2+ (Ramsay and Gadd, 1997). One explanation for this sensitivity is that the sequestration of Mn2+ into the vacuole relies on a proton-driven antiporter on the tonoplast.
Figure 4.
ShMTP1 Confers Mn2+ Tolerance to a Range of Mn2+-Sensitive Yeast Mutants.
Mutants of Saccharomyces strain BY4743 were transformed with the empty pYES2 vector (black bars) or with ShMTP1 in pYES2 (white bars). The ade1 strain was used as the control with a wild-type level of Mn2+ tolerance (A), whereas cnb (B), pmr1 (C), and vma8 (D) are Mn2+-sensitive mutants as described in the text. Cell growth was measured when cell density had reached ∼12 × 106 cells per mL after medium was inoculated to an initial density of 1.3 × 106 cells per mL. Data shown are means and standard errors (n = 3). Statistically significant differences in growth between yeast expressing ShMTP1 and yeast harboring the empty vector at a particular Mn2+ concentration are shown by single asterisks (P < 0.05) or double asterisks (P < 0.01) as determined by t test on data normalized to their respective 0-Mn2+ controls.
We confirmed that the mutants cnb1 and pmr1 were more sensitive to Mn2+ on both solid and liquid media (Figure 4), but the vma8 mutant showed a weaker Mn2+-sensitive phenotype that was apparent only on solid medium (data not shown). Expression of ShMTP1 conferred Mn2+ tolerance to the control strain, the pmr1 mutant, and the cnb1 mutant but not to the vma8 mutant (Figure 4). Indeed, in contrast to all other strains, ShMTP1 expression made the vma8 mutant more sensitive to 10 mM Mn2+ (Figure 4D). Expression of ShMTP1 also was unable to confer increased Mn2+ tolerance to a vph2 mutant (data not shown), another vacuolar acidification mutant with defects in the assembly of the V-type H+-ATPase (Jackson and Stevens, 1997). The requirement for a functional V-type H+-ATPase to obtain the Mn2+-tolerance phenotype conferred by ShMTP1 suggests that some direct or indirect reliance on a proton gradient is required for the functioning of this protein.
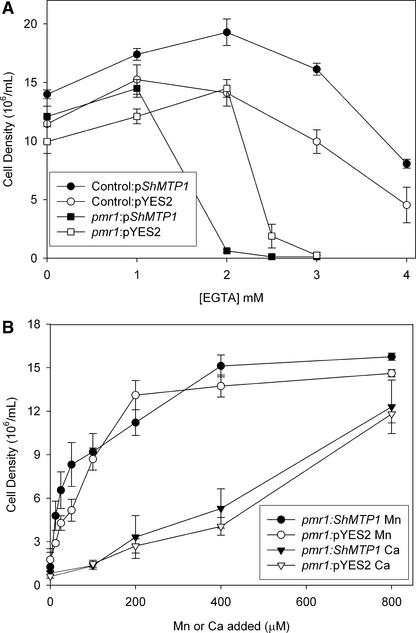
It is unlikely that ShMTP1 complements the functions defective in the cnb1 mutant, because wild-type CNB1 is a protein phosphatase and not a membrane-bound protein (Farcasanu et al., 1995). Therefore, it is more likely that ShMTP1 acts independently of CNB1 to confer Mn2+ tolerance by some other mechanism. Because ShMTP1 was able to confer a relatively high level of Mn2+ tolerance to the pmr1 mutant (Figure 4B), the mutant most sensitive to Mn2+ stress, and the PMR1 protein is able to transport Mn2+, it was possible that ShMTP1 complemented other functions defective in the pmr1 mutant. In addition to being sensitive to high external Mn2+ concentrations, pmr1 mutants are susceptible to Mn2+ and Ca2+ deficiency caused by EGTA (Wei et al., 2000). Both Ca2+ and Mn2+ are essential elements for yeast, and PMR1 is the primary mechanism for transport of the ions into the Golgi, where Mn2+ plays specific roles in the glycosylation of proteins and Ca2+ is involved in accurate sorting of proteins to the vacuole (Dürr et al., 1998). EGTA is an effective chelator of both Mn2+ and Ca2+ and is able to reduce the concentration of these ions to the cell and ultimately the Golgi complex, where they are required. Therefore, we tested the hypothesis that ShMTP1 was able to complement other functions of PMR1 in addition to conferring Mn2+ tolerance by assessing the EGTA sensitivity of pmr1 cells expressing ShMTP1. If expression of ShMTP1 were able to complement or partially complement the functions of the high-affinity Ca2+/Mn2+-ATPase, then the pmr1 mutant would be expected to regain tolerance to EGTA. However, ShMTP1 expression further increased the EGTA sensitivity of the pmr1 mutant, indicating that ShMTP1 did not complement the EGTA-sensitive phenotype of the pmr1 mutant (Figure 5A). The EGTA sensitivity of pmr1 cells was rescued by the external addition of Mn2+ but required higher Ca2+ concentrations to achieve a similar effect (Figure 5B).
Figure 5.
Expression of ShMTP1 Exacerbates the EGTA Sensitivity of the Saccharomyces pmr1 Mutant, Whereas Exogenously Added Mn2+ and Ca2+ Ameliorate the EGTA Sensitivity.
(A) Effect of EGTA on the growth of control (circles) and pmr1 mutant (squares) cells of Saccharomyces strain BY4743 either expressing ShMTP1 (closed symbols) or harboring the empty vector (open symbols). Cell growth was measured when the cell density had reached ∼12 × 106 cells per mL for the 0-EGTA controls after medium was inoculated to an initial density of 1.3 × 106 cells per mL. Data shown are means and standard errors (n = 3).
(B) Growth of the pmr1 mutant in the presence of EGTA was measured when cell density had reached ∼12 × 106 cells per mL for 0-EGTA controls after medium was inoculated to an initial density of 1.3 × 106 cells per mL. The medium contained 2.0 mM EGTA (pmr1:ShMTP1; closed symbols) or 2.75 mM EGTA (pmr1:pYES2; open symbols) supplemented with various Mn2+ (circles) or Ca2+ (inverted triangles) concentrations. The different EGTA concentrations used for the strains reflect their relative sensitivities to EGTA as shown in (A). Data shown are means and standard errors (n = 5 or 6).
Expression of ShMTP1 in Arabidopsis Confers Mn2+ Tolerance
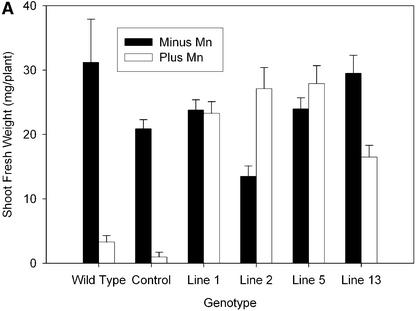
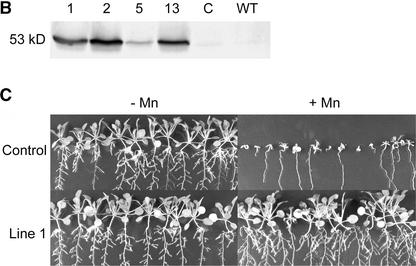
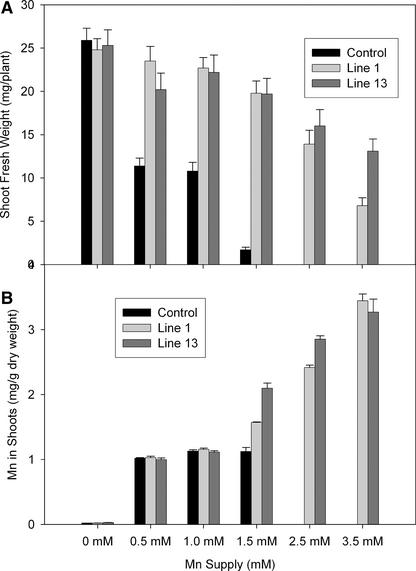
Expression of ShMTP1 in Arabidopsis conferred increased Mn2+ tolerance, as found for yeast. The increased tolerance in four independent homozygous lines expressing differing levels of ShMTP1 protein was shown by greater shoot weights compared with controls (Figures 6A and 6B). One line that had a relatively low shoot yield with basal Mn2+ showed a growth stimulation when supplied with excess Mn2+, suggesting that in this line, Mn2+ homeostasis was perturbed by the high-level expression of ShMTP1 (Figure 6A, line 2). The ability of ShMTP1 to protect seedlings from Mn2+ toxicity is illustrated in Figure 6C. In this experiment, the control seedlings died or grew poorly after germination on toxic Mn2+ concentrations, whereas the line expressing ShMTP1 showed robust growth on the same substrate. Two Arabidopsis lines expressing ShMTP1 were analyzed in greater detail for shoot yield and Mn accumulation over a range of Mn2+ concentrations (Figure 7). The two lines showed greater Mn2+ tolerance than the control at all Mn2+ levels and accumulated Mn in their shoots at concentrations similar to that in the control line with up to 1 mM Mn2+ in the substrate. At higher Mn2+ concentrations, the Mn2+-tolerant lines accumulated more Mn than the control line, and at >1.5 mM Mn2+, the control line either produced insufficient shoots for analysis or died soon after germination (Figure 7).
Figure 6.
ShMTP1 Expression Confers Mn2+ Tolerance to Arabidopsis.
(A) Shoot yield of Arabidopsis lines grown on nutrient agar for 16 days with basal Mn2+ (closed columns) or supplemented with 2.5 mM Mn2+ (open columns). The control is a homozygous transgenic line transformed with the empty vector, wild type indicates untransformed Arabidopsis, and lines 1, 2, 5, and 13 are a range of independent homozygous lines expressing ShMTP1. Data shown are means and standard errors (n = 25 to 34).
(B) Level of ShMTP1 expression in the various lines as determined with a specific antibody against ShMTP1. The identities of the lines are as described in (A); C indicates the control, and WT indicates the wild type. The molecular mass of ShMTP1 estimated from the immunoblot was marginally larger than that predicted from the sequence (47 kD).
(C) Growth of the control line compared with line 1 on agar containing basal or 2.5 mM Mn2+.
Figure 7.
Effect of ShMTP1 Expression on the Accumulation of Mn2+ in Arabidopsis Shoots.
(A) Shoot yield of the control and lines expressing ShMTP1 (lines 1 and 13) grown with a range of Mn2+ concentrations for 21 days. Data shown are means and standard errors (n = 50).
(B) Mn2+ concentration in shoots of the control and lines expressing ShMTP1 (lines 1 and 13) grown with a range of Mn2+ concentrations for 21 days. Data shown are means and standard errors for bulked samples from three Petri dishes (n = 3).
No data are shown for the control line at concentrations of >1.5 mM because of insufficient shoot material for analysis.
In a different experiment to measure accumulation over a shorter term, seedlings initially grown hydroponically with a basal Mn2+ concentration were exposed to 100 μM Mn2+ and Mn concentrations were determined in roots and shoots over a time course. The low Mn2+ concentration used in this experiment relative to the agar experiments reflects the binding of Mn2+ by components in the agar that effectively reduce the Mn2+ activity in solution. However, prolonged exposure (7 days) to this Mn2+ concentration proved toxic to the control plants. The accumulation of Mn in shoots and roots was similar for both control and ShMTP1-expressing plants over 7 days of Mn exposure (data not shown). These data indicate an internal tolerance of Mn2+ by the ShMTP1-expressing plants that is consistent with the sequestration of Mn2+ into an internal compartment.
ShMTP1 Is Located at the Plant Tonoplast
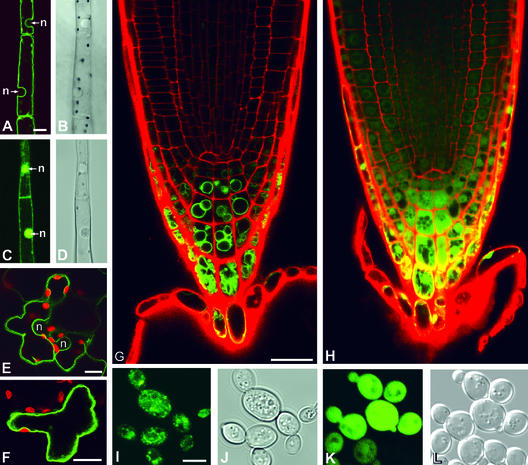
The presence of up to five membrane-spanning regions in ShMTP1 indicates that the protein is membrane bound, and TargetP (www.cbs.dtu.dk/services/TargetP/) predicted the chloroplast as the most likely location for ShMTP1. To localize ShMTP1 in plant cells, we prepared a construct that fused the green fluorescent protein (GFP) to the C terminus of the full ShMTP1 protein. Expression of the chimeric gene in tobacco and Arabidopsis showed that instead of in the chloroplast, as predicted by TargetP, ShMTP1 was located at the tonoplast, although no obvious tonoplast-targeting signal was evident in the sequence. Figure 8A shows that the fluorescence of the ShMTP1:GFP fusion protein in tobacco trichomes was associated with a discrete cellular region that for the most part tracked the cell wall, but the clear bulge that coincided with the presence of the nucleus (Figure 8B) was consistent with a tonoplast location for ShMTP1. Because these cells were heavily vacuolated, the presence of a signal that largely tracked the cell walls was expected, because the vacuole occupies most of the cell volume and the tonoplast would be pushed up against the plasma membrane, with only a thin layer of cytoplasm remaining between the membranes. Figure 8C shows the GFP-only control localizing to the cytoplasm. The thin layer of cytoplasm is visible, as is the strong fluorescence of the nucleus, which was the pattern observed by others when GFP was expressed without specific targeting signals attached (Haseloff et al., 1997).
Figure 8.
Confocal Microscopy Showing Fluorescence in Transgenic Tobacco, Arabidopsis, and Yeast Expressing an ShMTP1:GFP Fusion Protein.
(A) to (D) Tobacco trichomes expressing the ShMTP1:GFP fusion protein (A) or GFP alone (C) with the positions of the nuclei (n) shown. The corresponding light-field images are shown for trichromes expressing the ShMTP1:GFP fusion protein (B) or GFP alone (D). Bar in (A) = 10 μm; the same magnification is used for (B) to (D).
(E) and (F) Transient expression of ShMTP1:GFP in epidermal cells of tobacco leaves. Chloroplasts are indicated by the red autofluorescence, and the locations of nuclei (n) are indicated. Bars = 10 μm.
(G) and (H) Root tips of Arabidopsis expressing the ShMTP1:GFP fusion protein (G) or GFP alone (H). The red fluorescence is caused by cell walls stained with propidium iodide. Bar in (G) = 20 μm; the same magnification is used for (H).
(I) to (L) Fluorescence of yeast expressing ShMTP1:GFP (I) or GFP alone (K). The same cells visualized by Normaski optics are shown in (J) and (L), respectively. Bar in (I) = 4 μm; the same magnification is used for (J) to (L).
Similarly, in epidermal cells of tobacco leaves, the fluorescence showed bulges that were consistent with the presence of nuclei, although these were less obvious than those observed for the trichomes because of the irregular shape of the cells (Figure 8E). Chloroplasts present in the leaf cells provided additional evidence to support a tonoplast location for ShMTP1. In many cases, the green fluorescence from GFP was seen to skirt the red autofluorescence of the chloroplasts toward the inside of the cell and away from the cell wall (Figure 8F); based on reasoning similar to that described above for the nucleus, this finding is consistent with a tonoplast location for the protein. Furthermore, the simple structure of Arabidopsis roots allowed the meristematic tissues to be seen clearly. In young developing root cap cells, GFP-only expression showed relatively large cytoplasmic volume compared with that of older cells (Figure 8H). By contrast, the expression of ShMTP1:GFP showed young root cap cells with bubble-like structures that eventually occupied most of the volume of older cells (Figure 8G). These bubble-like structures likely represent the tonoplasts of developing vacuoles.
In contrast to its effect in plants, an ShMTP1:GFP fusion protein expressed in yeast did not show clear localization to the tonoplast. High-level expression under the control of the GAL1 promoter resulted in the accumulation of the fusion protein to internal membranes such as the endoplasmic reticulum that differed markedly from that of the GFP-only control (Figures 8I to 8L). The pattern was unlike those described for the plasma membrane (Panek et al., 2000), the tonoplast (Suriapranta et al., 2000), or the Golgi (Ton et al., 2002) but was similar to that shown for the endoplasmic reticulum (Benghezal et al., 2000). Although no discrete signal was apparent at the plasma membrane or the vacuolar membrane, the notion that a small proportion of the protein was incorporated into these membranes could not be discounted.
Expression of the ShMTP1:GFP fusion protein conferred Mn tolerance to both yeast and Arabidopsis, indicating that the attached GFP peptide did not interfere with ShMTP1 function (data not shown). During construction of the cDNA that encoded the fusion protein, one plasmid was prepared in which a PCR-generated error mutated the codon encoding an Arg residue at position 123 of ShMTP1 to an Ile residue. The protein mutated at this one residue did not confer Mn2+ tolerance when expressed in either yeast or Arabidopsis, even though the subcellular location in both cases was the same as that of the wild-type protein fused to GFP (data not shown). Although this Arg residue is present in only two of the MTP proteins shown in the alignment in Figure 1, it immediately precedes the first membrane-spanning domain and appears to be critical for ShMTP1 function.
DISCUSSION
The ShMTP1 gene encodes a protein of the CDF family that is likely to be an Mn2+ transporter. The close similarity of three other cDNAs from Stylosanthes that also confer Mn2+ tolerance to yeast as well as four putative proteins from Arabidopsis with a high degree of homology with these proteins suggests that members of this CDF subfamily have related functions. Expression of ShMTP1 in several yeast strains provided indirect evidence that the ShMTP1 protein acts to sequester Mn2+ to internal organelles. The enhanced Mn accumulation into cells expressing ShMTP1 at high external Mn2+ concentrations and the increased EGTA sensitivity of the pmr1 mutant expressing ShMTP1 both are consistent with internal Mn2+ sequestration. One interpretation of the EGTA sensitivity of pmr1 mutants is that they are more prone than wild-type cells to Mn deficiency as a result of restricted Mn2+ transport to the Golgi. ShMTP1 expression further exacerbated the susceptibility of pmr1 cells to EGTA, suggesting that Mn2+ supply to the Golgi was restricted in these cells. Consistent with this hypothesis is the observation that an increase in external Mn2+ concentration was able to overcome the growth inhibition by EGTA (Figure 5B). The ability of external Ca2+ to ameliorate EGTA sensitivity, albeit less effectively than Mn2+, suggests that Ca2+ is able to substitute for Mn2+ in critical processes or that at high concentrations it frees up Mn2+ that otherwise would be chelated by EGTA. The enhanced Mn2+ tolerance and the increased EGTA sensitivity of the pmr1 mutant expressing ShMTP1 indicate that ShMTP1 may act as a transporter to sequester Mn2+ from the cytosol to an organelle or organelles at the expense of the Golgi complex. Increased EGTA sensitivity might not be apparent in wild-type yeast expressing ShMTP1 if the high affinity of PMR1 for Mn2+ (Km of ∼20 nM [Mandal et al., 2000]) effectively outcompetes ShMTP1 for Mn2+.
ShMTP1 in yeast appeared to be associated primarily with the endoplasmic reticulum, suggesting that this, or some other internal organelle, is the site of Mn2+ sequestration. The endoplasmic reticulum can act as a store for metal ions, and recently, Clemens et al. (2002a) showed that a CDF protein of Schizosaccharomyces pombe located at the endoplasmic reticulum is involved in Zn accumulation to this organelle. The requirement of an active V-type H+-ATPase for effective Mn2+ tolerance to be conferred by ShMTP1 in yeast suggests that, as in plants, the tonoplast also is the site where a small proportion of the ShMTP1 functions as an Mn2+/H+ antiporter. Alternatively, because the V-type H+-ATPase is not restricted to the tonoplast (Forgac, 1999), it also is possible that the loss of this activity in other organelles, such as the endoplasmic reticulum, disrupts the proton gradient across the membranes and prevents ShMTP1 from functioning as an Mn2+/H+ antiporter. It is known that some vacuolar proteins are mistargeted in mutants of the V-type H+-ATPase (Robinson et al., 1988), which may explain the inability of ShMTP1 to confer Mn2+ tolerance in the vma8 and vph2 mutants. However, analysis of the ShMTP1: GFP fusion in the acidification mutants and wild-type yeast cells showed a similar distribution of fluorescence, indicating that the mutations did not affect the gross distribution of ShMTP1 (data not shown). Similarly, the distribution of ZRC1 was unaffected when expressed in acidification mutants, indicating that mistargeting of proteins is not a general phenomenon in these mutants (MacDiarmid et al., 2002). Evidence that some CDF proteins require a proton gradient to transport metals comes from MacDiarmid et al. (2002) and Guffanti et al. (2002), who showed that CDF proteins from yeast and Bacillus subtilis can act as Zn2+/H+ antiporters.
In plants, ShMTP1 was localized specifically at the tonoplast, suggesting that the sequestration of Mn2+ to the vacuole is the mechanism that confers Mn2+ tolerance. This finding indicates that the signal that targets ShMTP1 to the tonoplast in plants is not recognized effectively by yeast and illustrates the notion that plant proteins are not necessarily localized to the same compartment in yeast even when the apparent phenotypes resulting from their expression, such as tolerance to a metal, are similar in both organisms. We attempted to directly measure a transport function for ShMTP1 in membrane vesicles derived from both yeast and plants using 54Mn2+. However, we were unable to detect enhanced Mn2+ transport conferred by ShMTP1 using either cation gradients (Na+ and K+) or proton gradients as driving forces (data not shown). The inability to detect a transport function in vitro could indicate either that ShMTP1 is not an Mn2+ transporter or, more likely, that specific conditions in either the membrane preparations or the transport assays are required to maintain an active protein.
Overexpression of ShMTP1 conferred a clear increase in Mn2+ tolerance to Arabidopsis that was associated with an increased tolerance to internal Mn2+. Whether MTP-like genes can explain some of the natural variation observed in Mn2+ tolerance within plant species is not known. The observation that a range of species tolerate internal Mn rather than exclude Mn2+ (Horst, 1988) is consistent with a role for these genes in the Mn2+ tolerance of natural populations. Other genes that encode transporters capable of protecting plants from Mn2+ toxicity include CAX2 and ECA1. CAX2 encodes a protein located at the tonoplast that transports several metals, including Ca2+, Mn2+, and Cd2+ (Hirschi et al., 2000). Expression of CAX2 in tobacco conferred some tolerance to Mn2+, although this was not demonstrated as greater shoot weights. ECA1 encodes a Ca2+ pump that also is capable of transporting Zn2+ and Mn2+ to the endoplasmic reticulum (Wu et al., 2002). Arabidopsis seedlings mutated in ECA1 are sensitive to Mn2+, and although overexpression of ECA1 complements this phenotype, Mn2+ tolerance did not appear to be increased above that of wild-type seedlings.
The ShMTP1 gene encodes a membrane protein that differs from both CAX2 and ECA1, and in view of its ability to confer a strong Mn2+-tolerance phenotype to Arabidopsis, it may have applications in increasing the Mn2+ tolerance of selected plant species for agriculture by genetic manipulation. Mn2+ toxicity is one of the factors that limit plant production on acid soils, and some plant species, such as alfalfa, are particularly sensitive to the high Mn2+ concentrations that can occur in acidic soils (Foy, 1983). Furthermore, the increased tolerance of Mn2+ by sequestration to internal organelles allowed the transgenic plants to accumulate Mn2+ in shoots at concentrations that otherwise were toxic to nontransgenic plants. The ability to tolerate high internal concentrations of a heavy metal is one of the traits required for the genetic engineering of plants that are capable of hyperaccumulating metals (Clemens et al., 2002b). The development of fast-growing plants capable of hyperaccumulating heavy metals has applications in the bioremediation of sites contaminated with toxic metals.
METHODS
Yeast Strains and Culture
A Stylosanthes hamata cDNA library cloned into a modified pYES2 vector (Smith et al., 1995) was used to transform Saccharomyces cerevisiae strain INVSc2 (Invitrogen, Carlsbad, CA) (MATα his3-Δ1 ura3-52) using a method described by Gietz et al. (1995). Primary transformants were selected on medium that lacked uracil, washed off the plates with sterile water, and stored in 15% glycerol at −80°C until used. Putative Mn2+-tolerance genes were isolated by screening ∼106 cells on Petri plates that contained synthetic complete medium (Rose et al., 1990) supplemented with His, 40 mM MnCl2, and galactose to induce expression of the cloned cDNAs inserted behind the GAL1 promoter of pYES2. Plasmids from yeast colonies that grew on the medium were rescued in Escherichia coli (strain XL1-Blue; Stratagene, La Jolla, CA) and retransformed into the parental yeast strain to confirm that the Mn2+ tolerance was conferred by the plasmid. Other yeast strains used included a Ca2+-sensitive mutant (K667) (vcx1::hisG cnb1::LEU2 pmc1::TRP1 ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 [Cunningham and Fink, 1996]) and a range of Mn2+-sensitive mutants in the diploid strain BY4743 (MATa/a his3Δ1/his3Δ1 leu2Δ0 /leu2Δ0 lys2Δ0/LYS2 MET15/met15Δ0 ura3Δ 0/ura3Δ0). These mutants were generated by the Saccharomyces Genome Deletion Project (http://www-sequence.stanford.edu/group/yeast_deletion_project/) and included ade1 (used as a control with wild-type levels of Mn2+ tolerance; disrupted in YAR015W), cnb1 (disrupted in YKL190W), pmr1 (disrupted in YGL167C), vma8 (disrupted in YEL051W), and vph2 (disrupted in YKL119C). Detailed descriptions of these mutants can be obtained from the Saccharomyces Genome Database (http://genome-www.stanford.edu/Saccharomyces/). For growth experiments in liquid medium, the strains were cultured in synthetic complete medium supplemented with His, Lys, Leu, Trp, and adenine at the concentrations given by Rose et al. (1990).
Plant and Yeast Transformations
The ShMTP1 cDNA was ligated into the SalI-SphI site of the pDH51 expression cassette, which uses the 35S promoter and terminator of Cauliflower mosaic virus (Pietrzak et al., 1986). Subsequently, the EcoRI fragment containing the Stylosanthes cDNA was introduced into the EcoRI site of the binary vector pPLEX502 (Schünmann et al., 2003). Plant transformation vectors were transferred into Agrobacterium tumefaciens strain AGL1 by triparental mating. To localize ShMTP1, GFP was fused to the C terminus of ShMTP1. An in-frame fusion of GFP attached to the C terminus of ShMTP1 was prepared by PCR amplification of the coding sequence of ShMTP1 with the primers 5′-CTAAACGAATTCCCCGGGATGGACGCCAATTCGGG-3′ and 5′-CTCCACGAATTCTGA-CTGATTGTTGGGCAG-3′. The primers included EcoRI sites (underlined), and the reverse primer lacked a stop codon. The coding region of a GFP optimized for expression in plants was removed from pMng1004 (Upadhyaya et al., 1998) with NcoI and NotI, end-filled to yield a blunted fragment, and then inserted into the SmaI site of pART7 (Gleave, 1992) in the correct orientation with respect to the 35S promoter.
The ShMTP1 PCR product was digested with EcoRI and inserted into the EcoRI site of the pART7 vector upstream of and in-frame with the GFP coding region. The resulting cassette was removed as a NotI fragment, inserted into the NotI site of pPLEX502, and transferred to Agrobacterium as described above. A similar construct was prepared for the expression of a ShMTP1:GFP fusion protein in yeast except that the GFP was derived from the plasmid pCBJ4 (Benghezal et al., 2000), because the GFP version optimized for plant expression showed poor expression in yeast. The ShMTP1 cDNA that lacked a stop codon was inserted into the EcoRI site of pBC SK (Stratagene) with its 3′ end adjacent to the PstI site. The GFP coding region from pCBJ4 was amplified by PCR with the following primers that included PstI sites (underlined): 5′-AACTGCAGA-TGAGAGGAGAAGAACTTTTCAC-3′ and 5′-AACTGCAGTTATTTGTATAGTTCATCCATGC-3′. The resulting PCR product was digested with PstI and inserted into the PstI site with its 5′ end adjacent to the 3′ end of ShMTP1 in pBC SK. A NotI-SalI fragment containing the construct then was introduced into a modified version of pYES2 in which the KpnI site was converted to SalI (Smith et al., 1995), and yeast was transformed as described above.
The plant ShMTP1:GFP construct yielded a protein with the additional peptide EFGTP inserted between the ShMTP1 and GFP coding regions, whereas the yeast construct included the peptide EFLQ between the coding regions. Transient expression of the ShMTP1:GFP fusion in tobacco leaves was undertaken using an Agrobacterium infiltration method as described by Yang et al. (2000). Stable tobacco transformants were obtained subsequently by incubating the infiltrated leaves on Murashige and Skoog (1962) agar medium that contained 100 μg/mL kanamycin. Arabidopsis was transformed using the floral-dip technique (Clough and Bent, 1998) or by root transformation (Valvekens et al., 1988). Primary transformants were selected on nutrient agar medium (Richardson et al., 2000) supplemented with 50 μg/mL kanamycin. Subsequent generations also were selected on kanamycin to identify lines homozygous for the introduced genes. GFP expression in yeast and plant tissues was detected using a Leica SP2 confocal laser scanning microscope (Wetzlar, Germany). Roots were stained with 10 μg/mL propidium iodide before observation.
Protein Gel Blot Analysis
The region encoding most of the N-terminal hydrophilic region of ShMTP1 (amino acids 8 to 125) was amplified by PCR of the ShMTP1 coding region with the forward primer 5′-TTCGGATCCAAACATCAAG-3′ (BamHI site underlined) and the reverse primer 5′-AAACTGCAGTGG-AAATTCTCATTGGCTCTTTC-3′ (PstI site underlined). The PCR product was subcloned into the BamHI-PstI sites of the E. coli expression vector pQE-31 (Qiagen, Clifton Hill, Victoria, Australia) that incorporates a poly-His tag onto the N terminus of the expressed polypeptide. The growth of expression cultures included 2% (w/v) glycerol before induction to eliminate protein toxicity that can occur as a result of basal transcription. After overnight growth, a 1:10 dilution was made into broth that included 0.1% glycerol, and the culture was grown at 37°C until the OD600 reached 0.6. Expression of the polypeptide was induced by isopropylthio-β-galactoside (1 mM), and the culture was grown at 30°C for another 4 to 6 h. The protein was purified under denaturing conditions on a nickel affinity column according to the manufacturer's procedures (Qiagen). The purified protein was used to generate a polyclonal antibody in a rabbit, and the antibody was purified by passage through an affinity column using ShMTP1 as the ligand.
Conditions for immunoaffinity purification with cyanogen bromide–activated Sepharose 4B (Sigma) were according to Caughey et al. (1999) except that the antibody was eluted with only 0.1 M Gly, pH 2.9. For protein gel blot analysis, plant tissues were homogenized in an equal weight-to-volume ratio with 0.1 M EGTA that contained 0.25% (v/v) Tween 20. The extracts were centrifuged at 13,000g for 5 min and assayed for protein content (Bradford, 1976). Denaturing buffer was added to a sample that contained 100 μg of protein, and the sample was heated at 95°C for 5 min and then separated by SDS–denaturing protein electrophoresis. The proteins were transferred to nitrocellulose, and antigens were detected with the purified anti-ShMTP1 and a secondary antibody conjugated to alkaline phosphatase using methods described by Rerie et al. (1991).
Mn Assays
Yeast medium (250 mL) was inoculated with a saturated primary yeast culture to yield a cell density of ∼2.5 × 106 cells per mL (OD600 of ∼0.2). At various times after the addition of MnCl2 to 5 mM, subsamples (40 mL) were removed and placed on ice. The cells were collected by centrifugation (3000g for 3 min), resuspended in 2 mL of ice-cold 10 mM CaCl2 to desorb loosely bound Mn, and then transferred to small vials. After centrifugation (15,000g for 20 s), the cells were washed two more times with 2-mL aliquots of 10 mM CaCl2 and finally once with deionized water. The cells were ashed at 550°C overnight, and the residue was taken up in a mixture of 75 μL of nitric acid and 75 μL of hydrogen peroxide. After the samples were heated to 100°C for ∼15 min to clarify any residues, the volume was made up to 3 mL with deionized water. Plant shoots were collected, dried, and weighed before being ashed and prepared as described for the yeast. Roots were treated in the same manner after incubation in 10 mM CaCl2 for 1 h to desorb any loosely bound Mn. The Mn content of the digested samples was determined by atomic absorption spectrophotometry.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Accession Numbers
The GenBank accession numbers for the sequences described in this article are as follows: ShMTP1, AY181256; ShMTP2, AY181257; ShMTP3, AY181258; ShMTP4, AY181259; AtMTP1, NP_182203; AtMTP2, NP_ 191753; AtMTP3, NP_191440; AtMTP4, NP_180502; AtMTP5, NP_187817; AtMTP6, NP_182304; AtMTP7, NP_564594; AtMTP8, NP_191365; AtMTP9, NP_178070; AtMTP10, NP_173081; AtMTP11, NP_181477; AtMTP12, NP_178539; rice sequences, BAB67872, AAN52756.1, BAA993621.1; and Caenorhabditis elegans sequences, AAA81718.3, NP_509279.1, NP_504288.1, T16470, NP_498611.1, and T16640.
Acknowledgments
We thank Frank Smith for providing the Stylosanthes cDNA library, Narayana Upadhyaya for providing the plasmid pMng1004, Alan Richardson for assistance in Arabidopsis transformation, David Jones for providing plasmid pCBJ4, Kevin Gale and Malcolm Blundell for help in antibody production, and Richard Gardner and Kendal Hirschi for providing yeast strains.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.009134.
References
- Bartsevich, V.V., and Pakrasi, H.B. (1996). Manganese transport in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 271, 26057–26061. [DOI] [PubMed] [Google Scholar]
- Benghezal, M., Wasteneys, G.O., and Jones, D.A. (2000). The C-terminal dilysine motif confers endoplasmic reticulum localization to type I membrane proteins in plants. Plant Cell 12, 1179–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloß, T., Clemens, S., and Nies, D.H. (2002). Characterization of the ZAT1p zinc transporter from Arabidopsis thaliana in microbial model organisms and reconstituted proteoliposomes. Planta 214, 783–791. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Caughey, D.J., et al. (1999). Fractionation of polyclonal antibodies to fragments of a neuroreceptor using three increasingly chaotropic solvents. J. Chromatogr. B 728, 49–57. [DOI] [PubMed] [Google Scholar]
- Clemens, S., Bloss, T., Vess, C., Neumann, D., Nies, D.H., and zur Nieden, U. (2002. a). A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. J. Biol. Chem. 277, 18215–18221. [DOI] [PubMed] [Google Scholar]
- Clemens, S., Palmgren, M.G., and Krämer, U. (2002. b). A long way ahead: Understanding and engineering plant metal accumulation. Trends Plant Sci. 7, 309–315. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cragg, R.A., Christie, G.R., Phillips, S.R., Russi, R.M., Kury, S., Mathers, J.C., Taylor, P.M., and Ford, D. (2002). A novel zinc-regulated human zinc transporter, hZTL1, is localized to the enterocyte apical membrane. J. Biol. Chem. 277, 22789–22792. [DOI] [PubMed] [Google Scholar]
- Cunningham, K.W., and Fink, G.R. (1996). Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+-ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho, M.M., Andrew, C.S., Edwards, D.G., and Asher, C.J. (1980). Comparative performance of six Stylosanthes species in three acid soils. Aust. J. Agric. Res. 31, 61–76. [Google Scholar]
- Dürr, G., Strayle, J., Plemper, R., Elbs, S., Klee, S.K., Catty, P., Wolf, D.H., and Rudolph, H.K. (1998). The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9, 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcasanu, I.C., Hirata, D., Tsuchiya, E., Nishiyama, F., and Miyakawa, T. (1995). Protein phosphatase 2B of Saccharomyces cerevisiae is required for tolerance to manganese, in blocking the entry of ions into the cells. Eur. J. Biochem. 232, 712–717. [PubMed] [Google Scholar]
- Felsenstein, J. (1989). PHYLIP: Phylogeny Inference Package (version 3.2). Cladistics 5, 164–166. [Google Scholar]
- Forgac, M. (1999). Structure and properties of the vacuolar (H+)-ATPases. J. Biol. Chem. 274, 12951–12954. [DOI] [PubMed] [Google Scholar]
- Foy, C.D. (1983). The physiology of plant adaptation to mineral stress. Iowa State J. Res. 57, 355–392. [Google Scholar]
- Gietz, R.D., Schiestl, R.H., Willems, A.R., and Woods, R.A. (1995). Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11, 355–360. [DOI] [PubMed] [Google Scholar]
- Gleave, A.P. (1992). A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol. 20, 1203–1207. [DOI] [PubMed] [Google Scholar]
- Guffanti, A.A., Wei, Y., Rood, S.V., and Krulwich, T.A. (2002). An antiport mechanism for a member of the cation diffusion facilitator family: Divalent cations efflux in exchange for K+ and H+. Mol. Microbiol. 45, 145–153. [DOI] [PubMed] [Google Scholar]
- Haseloff, J., Siemering, K.R., Prasher, D.C., and Hodge, A. (1997). Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94, 2122–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi, K.D., Korenkov, V.V., Wilganowski, N.L., and Wagner, G.J. (2000). Expression of Arabidopsis CAX2 in tobacco: Altered metal accumulation and increased manganese tolerance. Plant Physiol. 124, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi, K.D., Zhen, R.-G., Cunningham, K.W., Rea, P.A., and Fink, G.R. (1996). CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc. Natl. Acad. Sci. USA 93, 8782–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst, W.J. (1988). The physiology of manganese toxicity. In Manganese in Soils and Plants, R.D. Graham, J. Hannam, and N.C. Uren, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 175–188.
- Jackson, D.D., and Stevens, T.H. (1997). VMA12 encodes a yeast endoplasmic reticulum protein required for vacuolar H+-ATPase assembly. J. Biol. Chem. 272, 25928–25934. [DOI] [PubMed] [Google Scholar]
- Lapinskas, P.J., Cunningham, K.W., Liu, X.F., Fink, G.R., and Culotta, V.C. (1995). Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15, 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., and Kaplan, J. (1998). Defects in the yeast high affinity iron transport system result in increased metal sensitivity because of the increased expression of transporters with a broad transition metal specificity. J. Biol. Chem. 273, 22181–22187. [DOI] [PubMed] [Google Scholar]
- Loukin, S., and Kung, C. (1995). Manganese effectively supports yeast cell-cycle progression in place of calcium. J. Cell Biol. 131, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDiarmid, C.W., Milanick, M.A., and Eide, D.J. (2002). Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J. Biol. Chem. 277, 39187–39194. [DOI] [PubMed] [Google Scholar]
- Mandal, D., Woolf, T.B., and Rao, R. (2000). Manganese selectivity of Pmr1, the yeast secretory pathway ion pump, is defined by residue Gln783 in transmembrane segment 6. J. Biol. Chem. 275, 23933–23938. [DOI] [PubMed] [Google Scholar]
- Mäser, P., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Panek, H.R., Conibear, E., Bryan, J.D., Colvin, R.T., Goshorn, C.D., and Robinson, L.C. (2000). Identification of Rgp1p, a novel Golgi recycling factor, as a protein required for efficient localization of yeast casein kinase 1 to the plasma membrane. J. Cell Sci. 113, 4545–4555. [DOI] [PubMed] [Google Scholar]
- Paulsen, I.T., and Saier, M.H. (1997). A novel family of ubiquitous heavy metal ion transport proteins. J. Membr. Biol. 156, 99–103. [DOI] [PubMed] [Google Scholar]
- Persans, M.W., Nieman, K., and Salt, D.E. (2001). Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proc. Natl. Acad. Sci. USA 98, 9995–10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak, M., Shillito, R.D., Hohn, T., and Potrykus, I. (1986). Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucleic Acids Res. 14, 5857–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay, L.M., and Gadd, G.M. (1997). Mutants of Saccharomyces cerevisiae defective in vacuolar function confirm a role for the vacuole in toxic metal ion detoxification. FEMS Microbiol. Lett. 152, 293–298. [DOI] [PubMed] [Google Scholar]
- Rerie, W.G., Whitecross, M., and Higgins, T.J.V. (1991). Developmental and environmental regulation of pea legumin genes in transgenic tobacco. Mol. Gen. Genet. 225, 148–157. [DOI] [PubMed] [Google Scholar]
- Richardson, A.E., Hadobas, P.A., and Hayes, J.E. (2000). Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant Cell Environ. 23, 397–405. [Google Scholar]
- Robinson, J.S., Klionsky, D.J., Banta, L.M., and Emr, S.D. (1988). Protein sorting in Saccharomyces cerevisiae: Isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8, 4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, M.D., Winston, F., and Hieter, P. (1990). Methods in Yeast Genetics: A Laboratory Course Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schünmann, P.H.D., Llewellyn, D.J., Surin, B., Boevink, P., De Feyter, R.C., and Waterhouse, P.M. (2003). A suite of novel promoters and terminators for plant biotechnology. Funct. Plant Biol., in press. [DOI] [PubMed]
- Smith, F.W., Ealing, P.M., Hawkesford, M.J., and Clarkson, D.T. (1995). Plant members of a family of sulfate transporters reveal functional subtypes. Proc. Natl. Acad. Sci. USA 92, 9373–9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriapranta, I., Epple, U.D., Bernreuther, D., Bredschneider, M., Sovarasteanu, K., and Thumm, M. (2000). The breakdown of autophagic vesicles inside the vacuole depends on Aut4p. J. Cell Sci. 113, 4025–4033. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton, V.-K., Mandal, D., Vahadji, C., and Rao, R. (2002). Functional expression in yeast of the human secretory pathway Ca2+,Mn2+-ATPase defective in Hailey-Hailey disease. J. Biol. Chem. 277, 6422–6427. [DOI] [PubMed] [Google Scholar]
- Upadhyaya, N.M., Ramm, K., Gaudron, J., Craig, S., Wang, M.B., Gupta, S., Okita, T.W., and Waterhouse, P.M. (1998). Can gfp replace uidA as a reporter gene to monitor transformation of cereals by biolistics or Agrobacterium? In Agriculture Biotechnology: Laboratory, Field and Market. Proceedings of the 4th Asia-Pacific Conference on Agricultural Biotechnology, Darwin, P.J. Larkin, ed (Canberra, Australia: Under The Counter Publishing), pp. 111–113.
- van der Zaal, B.J., Neuteboom, L.W., Pinas, J.E., Chardonnens, A.N., Schat, H., Verkleij, J.A.C., and Hooykaas, P.J.J. (1999). Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol. 119, 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens, D., Montagu, M.V., and Lijsebettens, M.V. (1988). Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. USA 85, 5536–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y., Chen, J., Rosas, G., Tompkins, D.A., Holt, P.A., and Rao, R. (2000). Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J. Biol. Chem. 275, 23927–23932. [DOI] [PubMed] [Google Scholar]
- Wu, Z., Liang, F., Hong, B., Young, J.C., Sussman, M.R., Harper, J.F., and Sze, H. (2002). An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol. 130, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T., and Forgac, M. (2000). Subunit D (Vma8p) of the yeast vacuolar H+-ATPase plays a role in coupling of proton transport and ATP hydrolysis. J. Biol. Chem. 275, 22075–22081. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Li, R., and Qi, M. (2000). In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 22, 543–551. [DOI] [PubMed] [Google Scholar]