Abstract
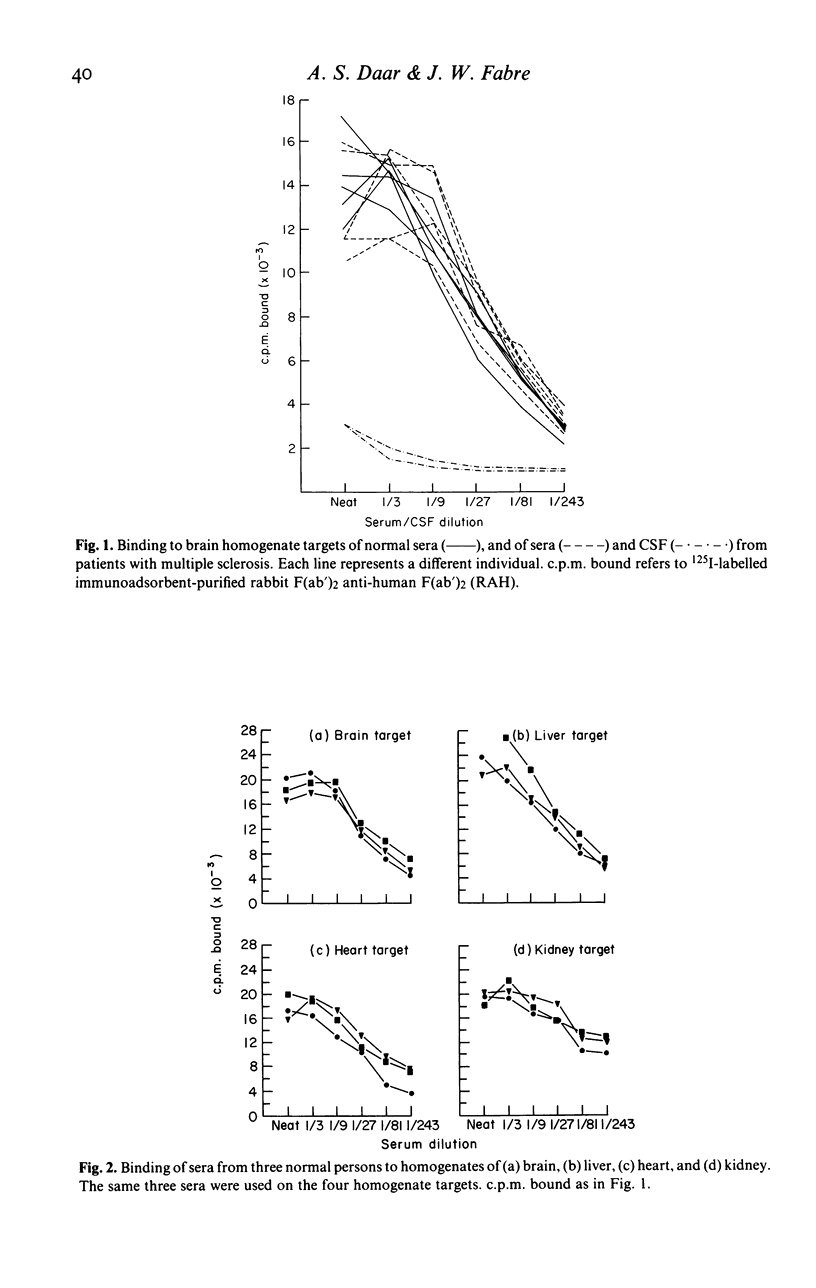
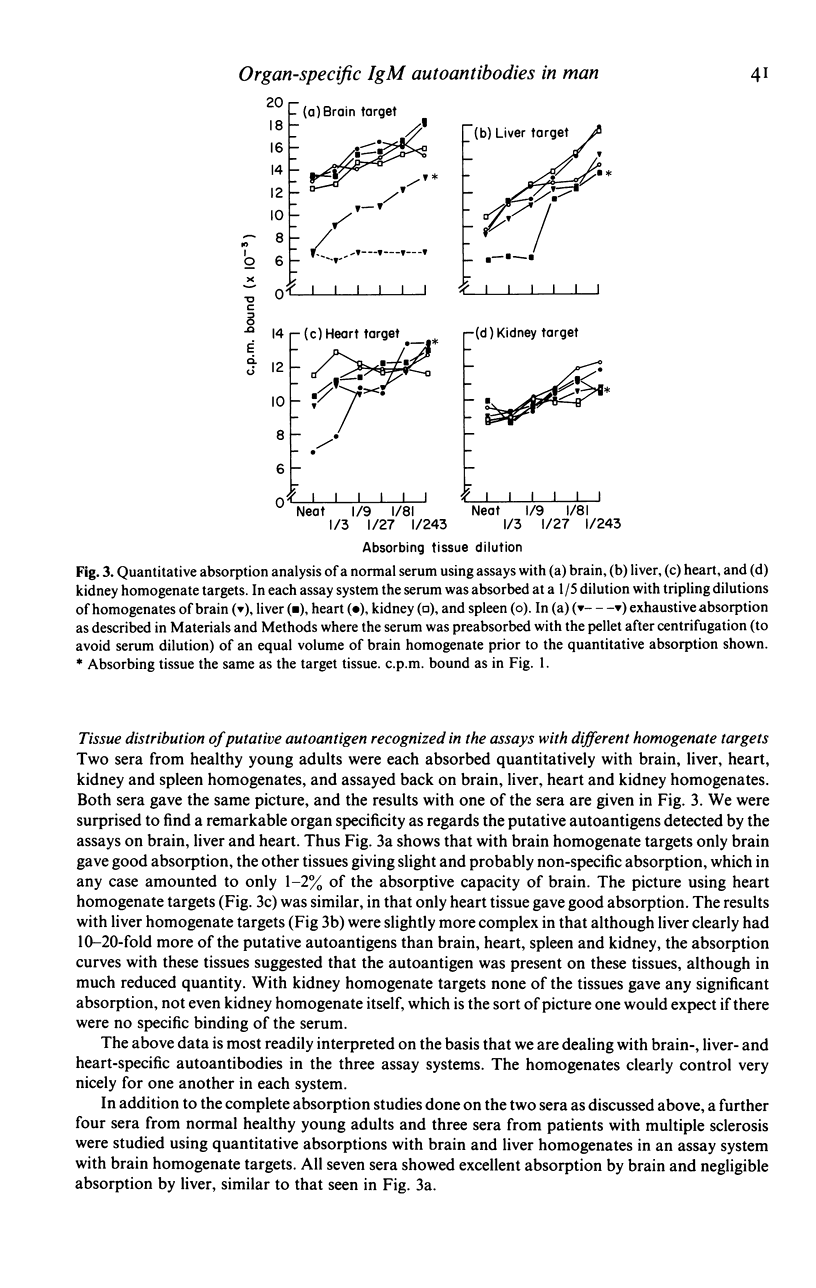
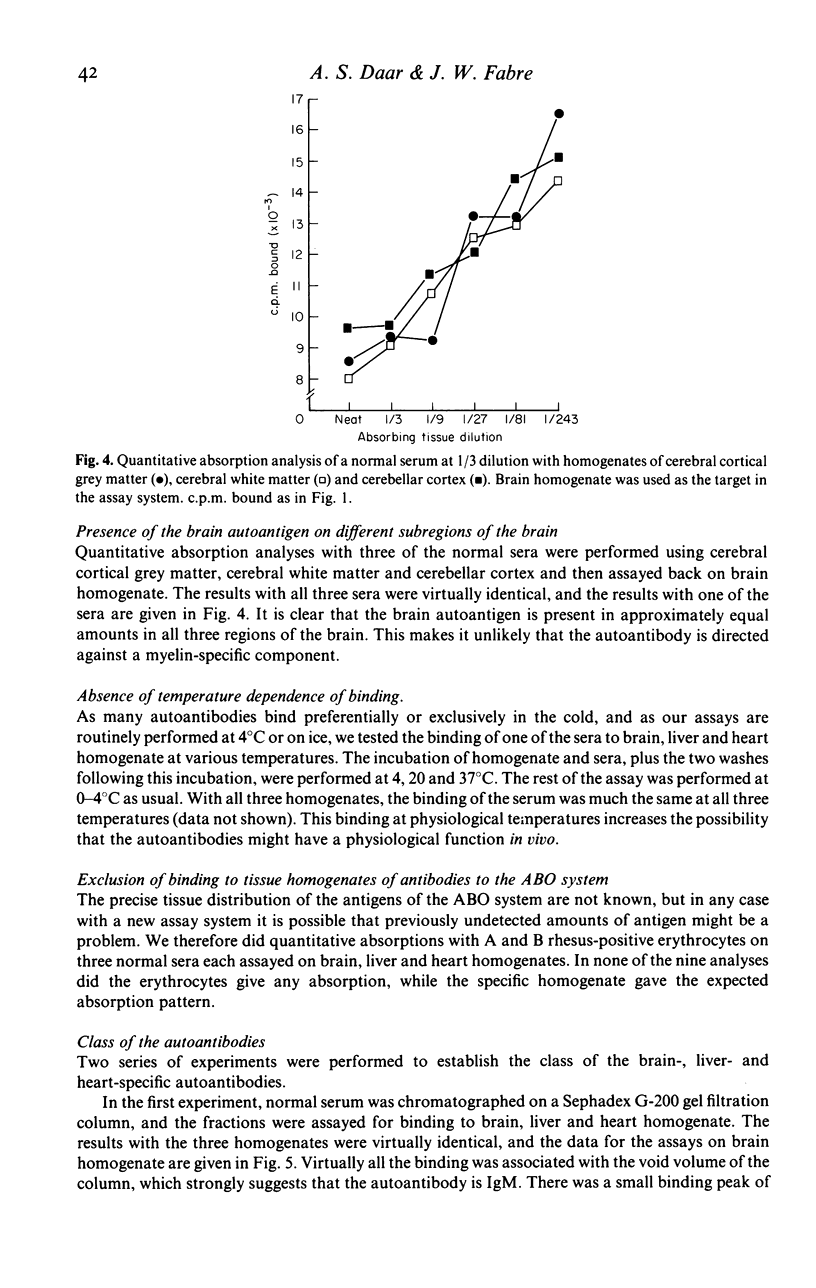
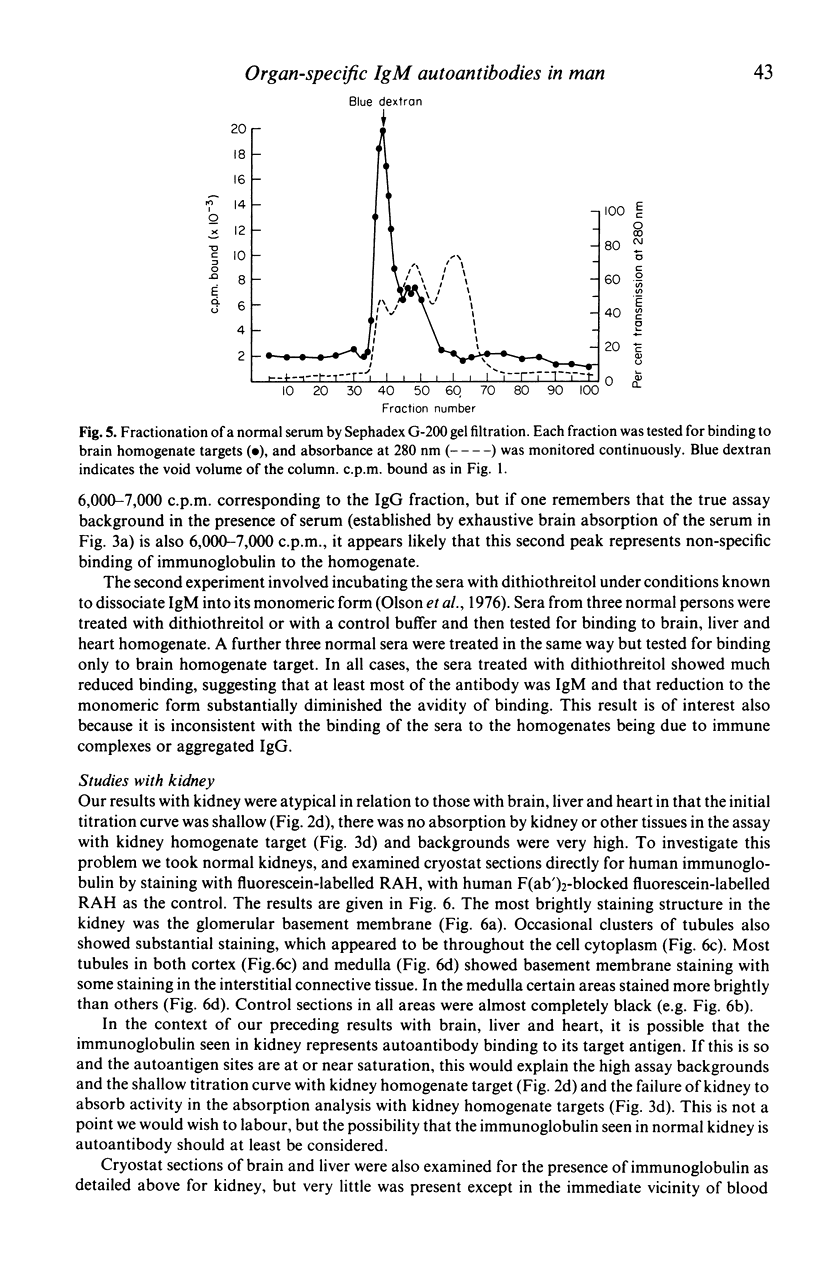
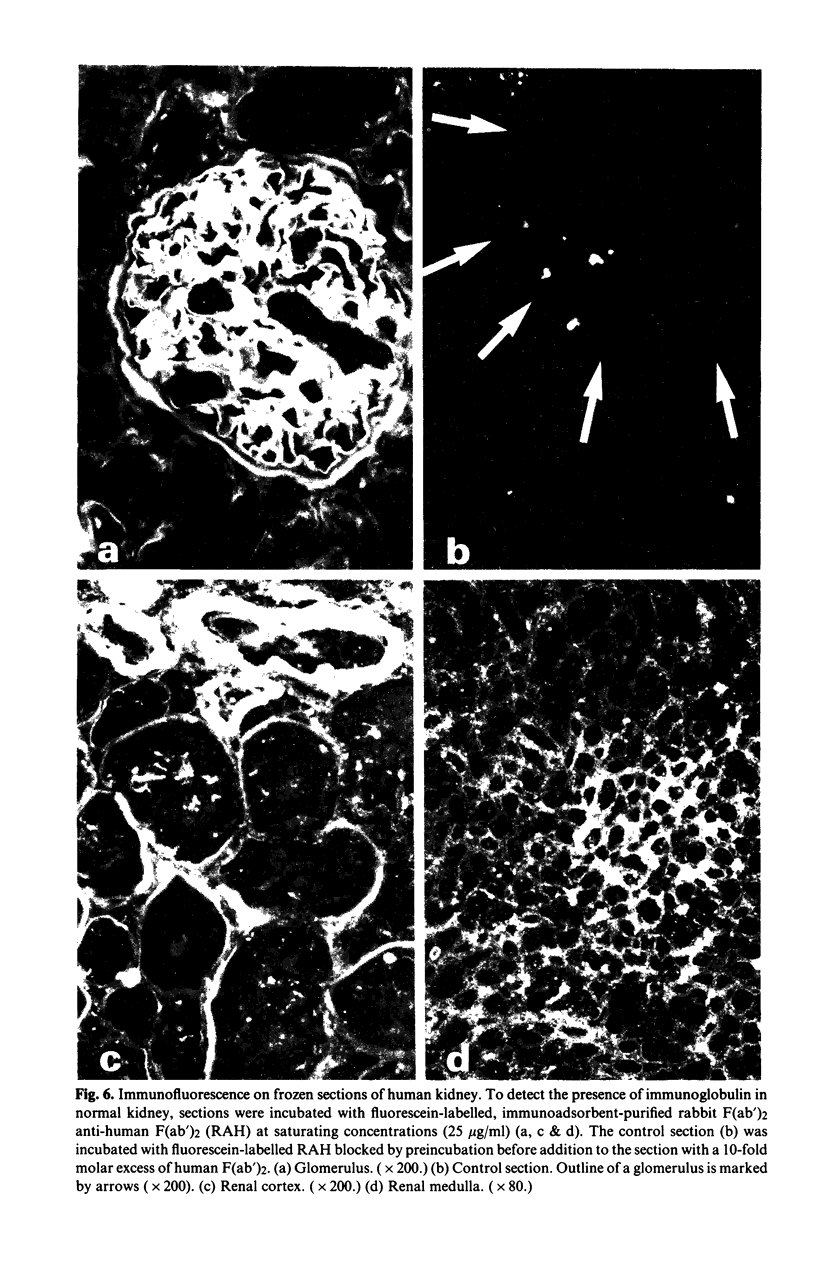
In this paper we use a sensitive, 125I-anti-immunoglobulin-binding assay, recently adapted for use with tissue homogenates as targets, to demonstrate autoantibodies to brain, liver and heart in the sera of normal persons. Quantitative absorption analyses demonstrated that the autoantigens detected were in each case organ-specific, and the brain autoantigen was shown to be present in equal amounts on cerebral cortex, cerebral white matter and cerebellar cortex. The autoantibodies were shown to be IgM in nature by gel filtration studies and experiments where IgM was reduced to monomers, and were found to bind equally well at 4, 20 and 37 degrees C. Cross-reactions with brain, liver and heart of rat and dog were unpredictable and usually weak. Parallel studies with kidney homogenates failed to detect anti-kidney autoantibodies, but immunofluorescence studies on frozen sections revealed large amounts of immunoglobulin in normal kidneys, mainly on glomerular and tubular basement membranes, raising the possibility that autoantibodies to kidney are present but that the autoantigen sites are situated in vivo. Sera from patients with multiple sclerosis were indistinguishable from normal sera in their binding to brain homogenate, and CSF from five patients with multiple sclerosis did not bind at all to brain homogenate. The theoretical and practical significance of multiple IgM autoantibodies in normal persons, and the organ specificity of the autoantibodies, is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramsky O., Lisak R. P., Silberberg D. H., Pleasure D. E. Antibodies to oligodendroglia in patients with multiple sclerosis. N Engl J Med. 1977 Dec 1;297(22):1207–1211. doi: 10.1056/NEJM197712012972204. [DOI] [PubMed] [Google Scholar]
- Allison A. C. Unresponsiveness to self antigens. Lancet. 1971 Dec 25;2(7739):1401–1403. doi: 10.1016/s0140-6736(71)90673-8. [DOI] [PubMed] [Google Scholar]
- Boss J. H., Silber E., Nelken D. Antibodies to species homologous tissue antigens in the rat. I. Naturally occurring circulating antibodies. Clin Exp Immunol. 1967 Mar;2(2):191–202. [PMC free article] [PubMed] [Google Scholar]
- Bullock J. Y., Booth R. J., Wilson J. D. Tissue antibodies in a healthy New Zealand population. N Z Med J. 1979 Jan 10;89(627):11–13. [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W 3/13 antigen of the rat. Eur J Immunol. 1980 Oct;10(10):745–749. doi: 10.1002/eji.1830101004. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Elson C. J., Naysmith J. D., Taylor R. B. B-cell tolerance and autoimmunity. Int Rev Exp Pathol. 1979;19:137–203. [PubMed] [Google Scholar]
- Fudenberg H. H. Genetically determined immune deficiency as the predisposing cause of "autoimmunity" and lymphoid neoplasia. Am J Med. 1971 Sep;51(3):295–298. doi: 10.1016/0002-9343(71)90263-4. [DOI] [PubMed] [Google Scholar]
- Grabar P. Hypothesis. Auto-antibodies and immunological theories: an analytical review. Clin Immunol Immunopathol. 1975 Nov;4(4):453–466. doi: 10.1016/0090-1229(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Kidney-specific allo- and autoantibodies in the alloantibody response to rat kidney: the use of kidney homogenate as a target for serological analysis. Clin Exp Immunol. 1980 Apr;40(1):111–119. [PMC free article] [PubMed] [Google Scholar]
- Hellström I., Hellström K. E. Can "blocking" serum factors protect against autoimmunity? Nature. 1972 Dec 22;240(5382):471–473. doi: 10.1038/240471a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morris R. J., Williams A. F. Antigens on mouse and rat lymphocytes recognized by rabbit antiserum against rat brain: the quantitative analysis of a xenogeneic antiserum. Eur J Immunol. 1975 Apr;5(4):274–281. doi: 10.1002/eji.1830050412. [DOI] [PubMed] [Google Scholar]
- Olson P. R., Weiblen B. J., O'Leary J. J., Moscowitz A. J., McCullough J. A simple technique for the inactivation of IgM antibodies using dithiothreitol. Vox Sang. 1976;30(2):149–159. doi: 10.1111/j.1423-0410.1976.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Standring R., McMaster W. R., Sunderland C. A., Williams A. F. The predominant heavily glycosylated glycoproteins at the surface of rat lymphoid cells are differentiation antigens. Eur J Immunol. 1978 Dec;8(12):832–839. doi: 10.1002/eji.1830081203. [DOI] [PubMed] [Google Scholar]
- Weinberger J. Z., Germain R. N., Benacerraf B., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. V. Role of idiotypes in the suppressor pathway. J Exp Med. 1980 Jul 1;152(1):161–169. doi: 10.1084/jem.152.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]


