Abstract
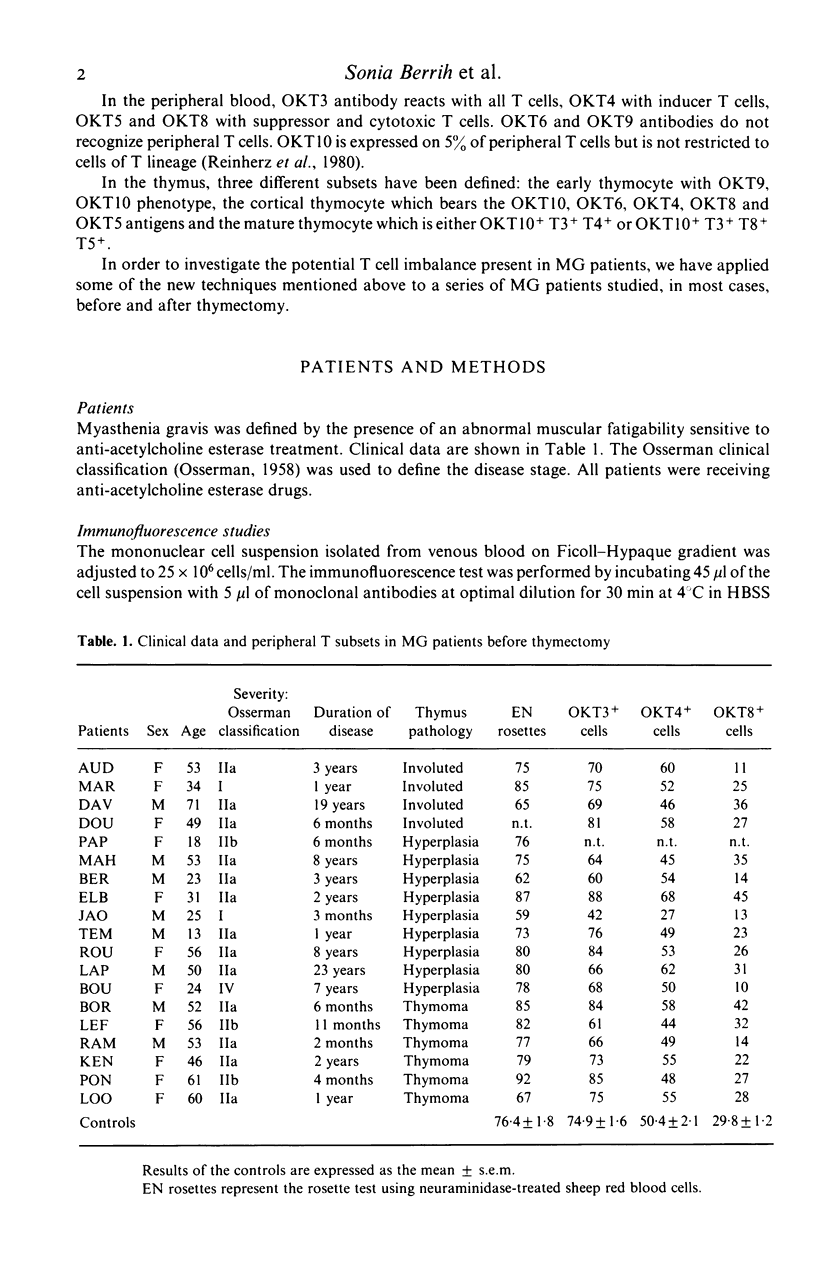
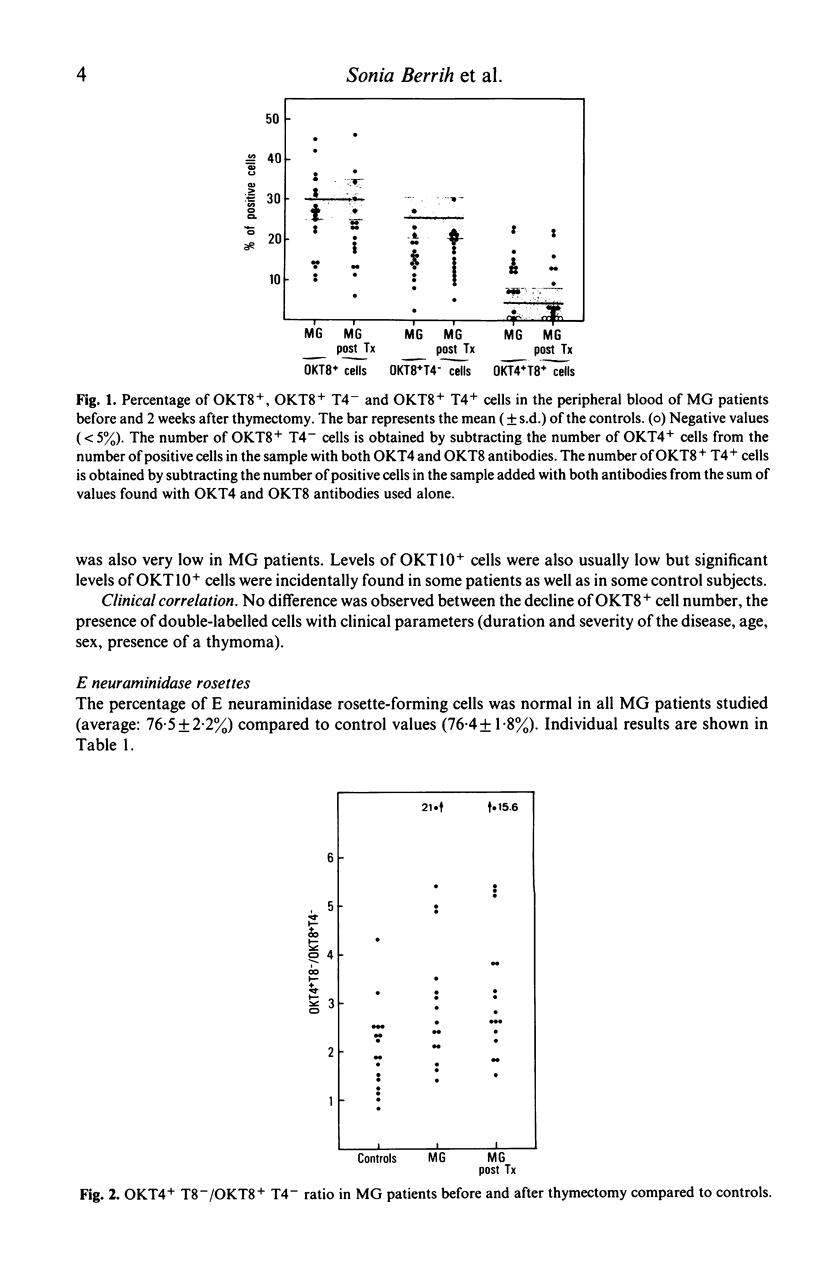
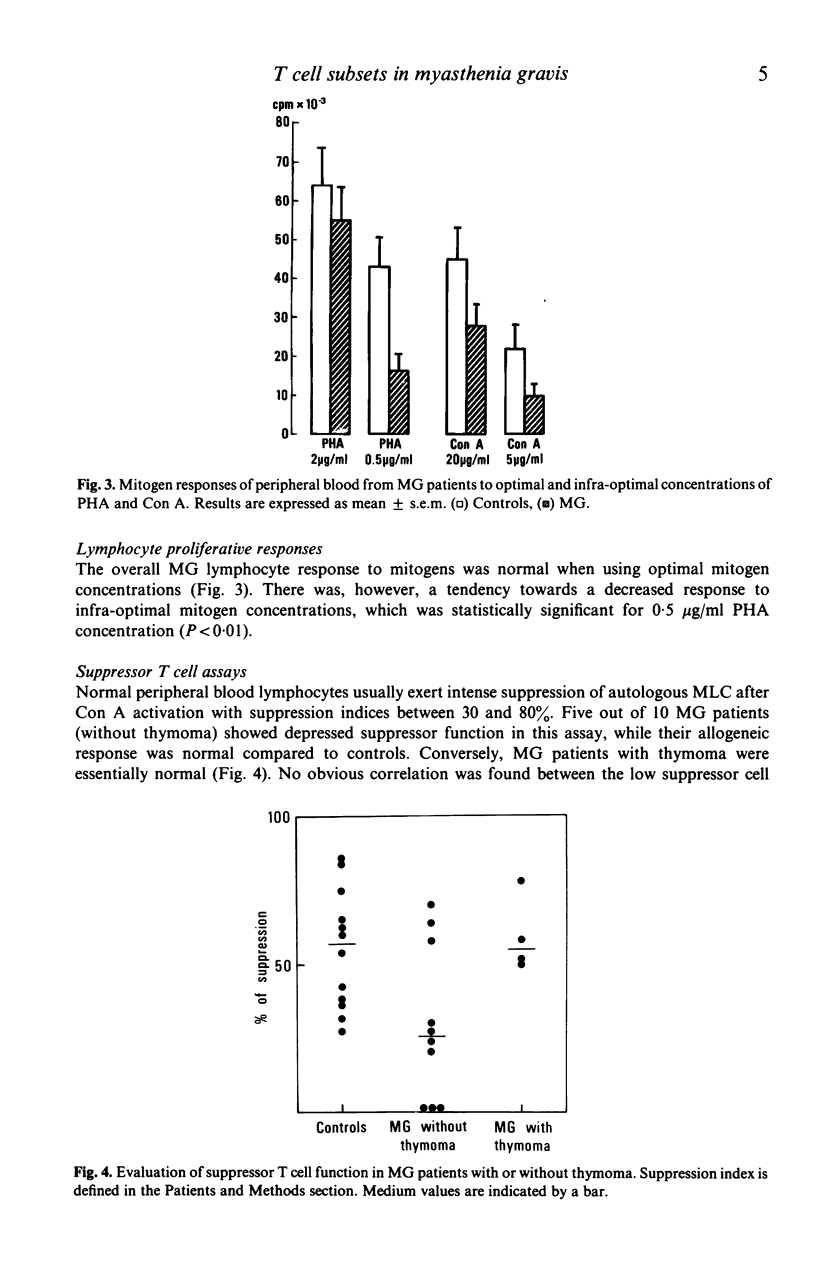
Functional T cell subsets have been evaluated in the peripheral blood of patients with myasthenia gravis using monoclonal anti-T cell antibodies and a suppressor cell assay based on the suppression of the mixed-lymphocyte reaction by concanavalin A-activated lymphocytes. A significant decline of suppressor cells was found in a large proportion of patients, both by direct count using the anti-suppressor-cytotoxic T cell antibody (OKT8) and by the suppressor assay. Patients also showed an increase in immature T cells defined by their simultaneous reaction with the anti-helper cell (OKT4) and anti-suppressor cell (OKT8) antibody. Thymectomy tended to enhance the deficit in suppressor cells, and to induce the disappearance of double-labelled cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Lisak R. P., Zweiman B., Abrahamsohn I., Penn A. S. The thymus in myasthenia gravis. Evidence for altered cell populations. N Engl J Med. 1974 Dec 12;291(24):1271–1275. doi: 10.1056/NEJM197412122912403. [DOI] [PubMed] [Google Scholar]
- Bach J. F. Evaluation of T-cells and thymic serum factors in man using the rosette technique. Transplant Rev. 1973;16(0):196–217. doi: 10.1111/j.1600-065x.1973.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum G., Tsairis P. Thymic lymphocytes in myasthenia gravis. Ann Neurol. 1977 Apr;1(4):331–333. doi: 10.1002/ana.410010404. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csapo A. I., Pohanka O., Kaihola H. L. Progesterone deficiency and premature labour. Br Med J. 1974 Jan 26;1(5899):137–140. doi: 10.1136/bmj.1.5899.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Angus C. W., Adams R. N., Kao I. Effect of myasthenic patients' immunoglobulin on acetylcholine receptor turnover: selectivity of degradation process. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3422–3426. doi: 10.1073/pnas.75.7.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze D., Herrman C., Jr, Naeim F., Smith G. S., Walford R. L. HL-A antigens in myasthenia gravis. Lancet. 1974 Feb 16;1(7851):240–242. doi: 10.1016/s0140-6736(74)92548-3. [DOI] [PubMed] [Google Scholar]
- Galili U., Schlesinger M. The formation of stable E rosettes after neuraminidase treatment of either human peripheral blood lymphocytes or of sheep red blood cells. J Immunol. 1974 May;112(5):1628–1634. [PubMed] [Google Scholar]
- Goldstein G., Manganaro A. Thymin: a thymic polypeptide causing the neuromuscular block of myasthenia gravis. Ann N Y Acad Sci. 1971 Sep 15;183:230–240. doi: 10.1111/j.1749-6632.1971.tb30754.x. [DOI] [PubMed] [Google Scholar]
- Huang S. W., Rose J. W., Mayer R. F. Assessment of cellular and humoral immunity of myasthenics. J Neurol Neurosurg Psychiatry. 1977 Nov;40(11):1053–1059. doi: 10.1136/jnnp.40.11.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoyama Y., Kawanami S., Goto I., Kuroiwa Y. T and B lymphocytes in myasthenia gravis. Clin Exp Immunol. 1979 Sep;37(3):452–456. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Enhanced immune responsiveness to a thymus-independent antigen early after adult thymectomy: evidence for short-lived inhibitory thymus-derived cells. Eur J Immunol. 1972 Apr;2(2):114–118. doi: 10.1002/eji.1830020204. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Le Brigand H., Leuasseur P., Merlier M., Rojas-Miranda A., Gaud C., Noviant Y. Cent thymectomies chez des myasthéniques. Résultats lointains. Rev Neurol (Paris) 1972 Apr;126(4):267–274. [PubMed] [Google Scholar]
- Opelz G., Keesey J., Glovsky M. M., Gale R. P. Autoreactivity between lymphocytes and thymus cells in myasthenia gravis. Arch Neurol. 1978 Jul;35(7):413–415. doi: 10.1001/archneur.1978.00500310015003. [DOI] [PubMed] [Google Scholar]
- Papatestas A. E., Alpert L. I., Osserman K. E., Osserman R. S., Kark A. E. Studies in myasthenia gravis: effects of thymectomy. Results on 185 patients with nonthymomatous and thymomatous myasthenia gravis, 1941-1969. Am J Med. 1971 Apr;50(4):465–474. doi: 10.1016/0002-9343(71)90336-6. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Richman D. P., Patrick J., Arnason B. G. Cellular immunity in myasthenia gravis. Response to purified acetylcholine receptor and autologous thymocytes. N Engl J Med. 1976 Mar 25;294(13):694–698. doi: 10.1056/NEJM197603252941304. [DOI] [PubMed] [Google Scholar]
- Rotter V., Trainin N. Thymus cell population exerting a regulatory function in the immune response of mice to polyvinyl pyrrolidone. Cell Immunol. 1974 Jul;13(1):76–86. doi: 10.1016/0008-8749(74)90228-7. [DOI] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Stanley E. F., Drachman D. B. Effect of myasthenic immunoglobulin on acetylcholine receptors of intact mammalian neuromuscular junctions. Science. 1978 Jun 16;200(4347):1285–1287. doi: 10.1126/science.663610. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Mickey M. R. HL-A haplotypes of 32 diseases. Transplant Rev. 1975;22:105–119. doi: 10.1111/j.1600-065x.1975.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Wijermans P., Oosterhuis H. J., Astaldi G. C., Schellekens P. T., Astaldi A. Influence of adult thymectomy on immunocompetence in patients with myasthenia gravis. J Immunol. 1980 Apr;124(4):1977–1982. [PubMed] [Google Scholar]
- Zilko P. J., Dawkins R. L., Holmes K., Witt C. Genetic control of suppressor lymphocyte function in myasthenia gravis: relationship of impaired suppressor function to HLA-B8/DRW3 and cold reactive lymphocytotoxic antibodies. Clin Immunol Immunopathol. 1979 Oct;14(2):222–230. doi: 10.1016/0090-1229(79)90143-0. [DOI] [PubMed] [Google Scholar]


