Abstract
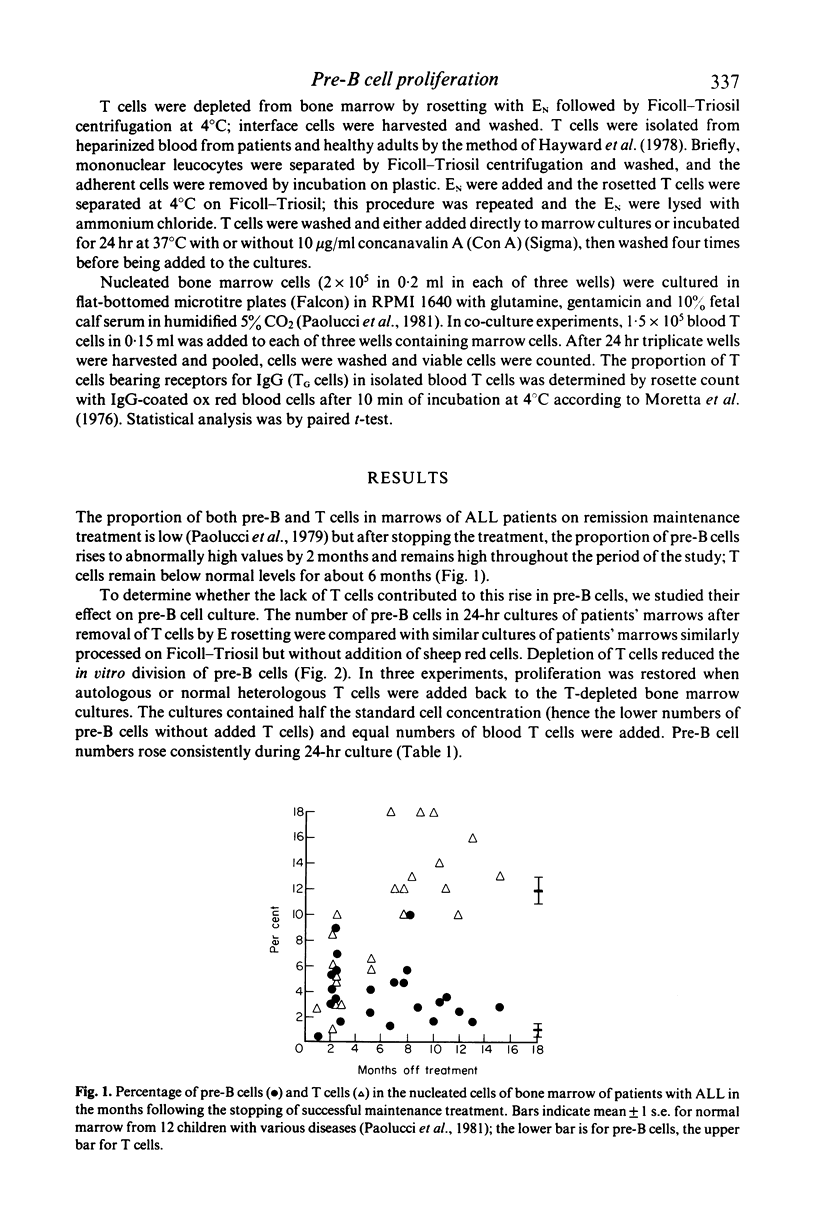
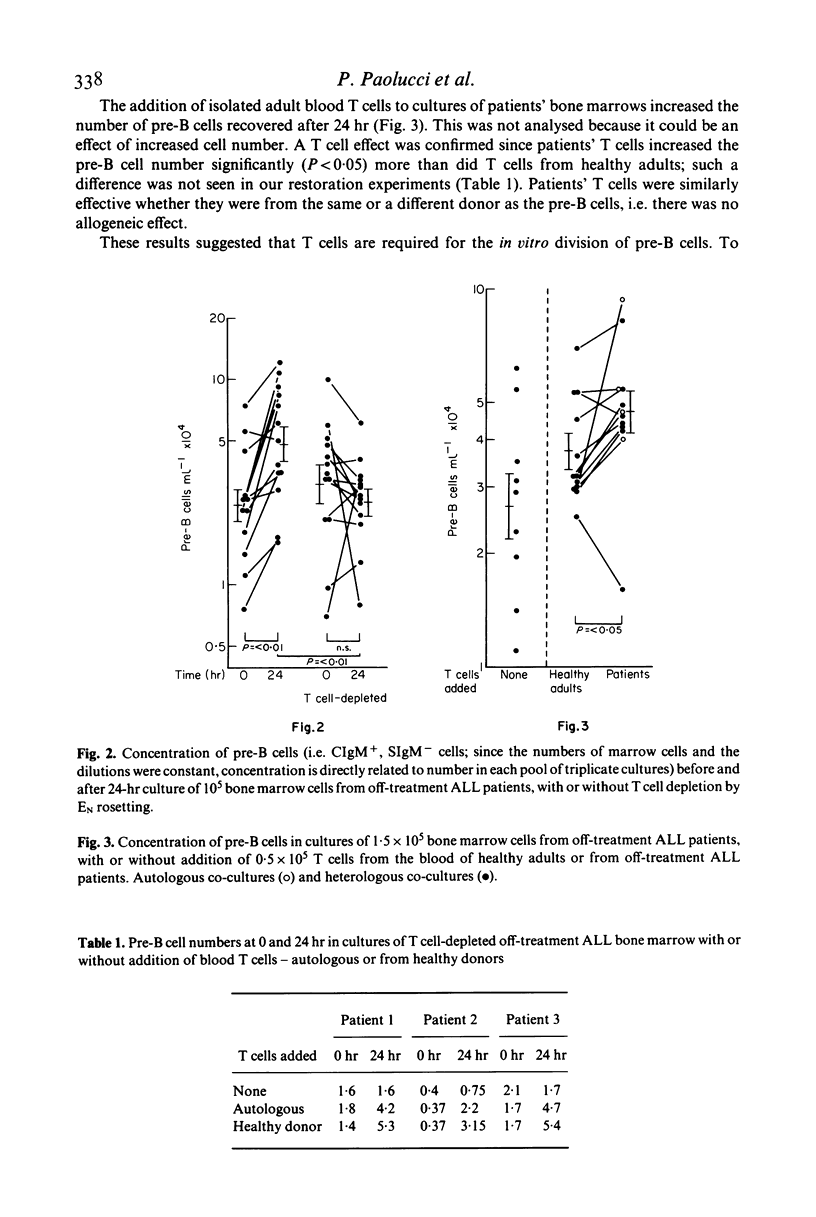
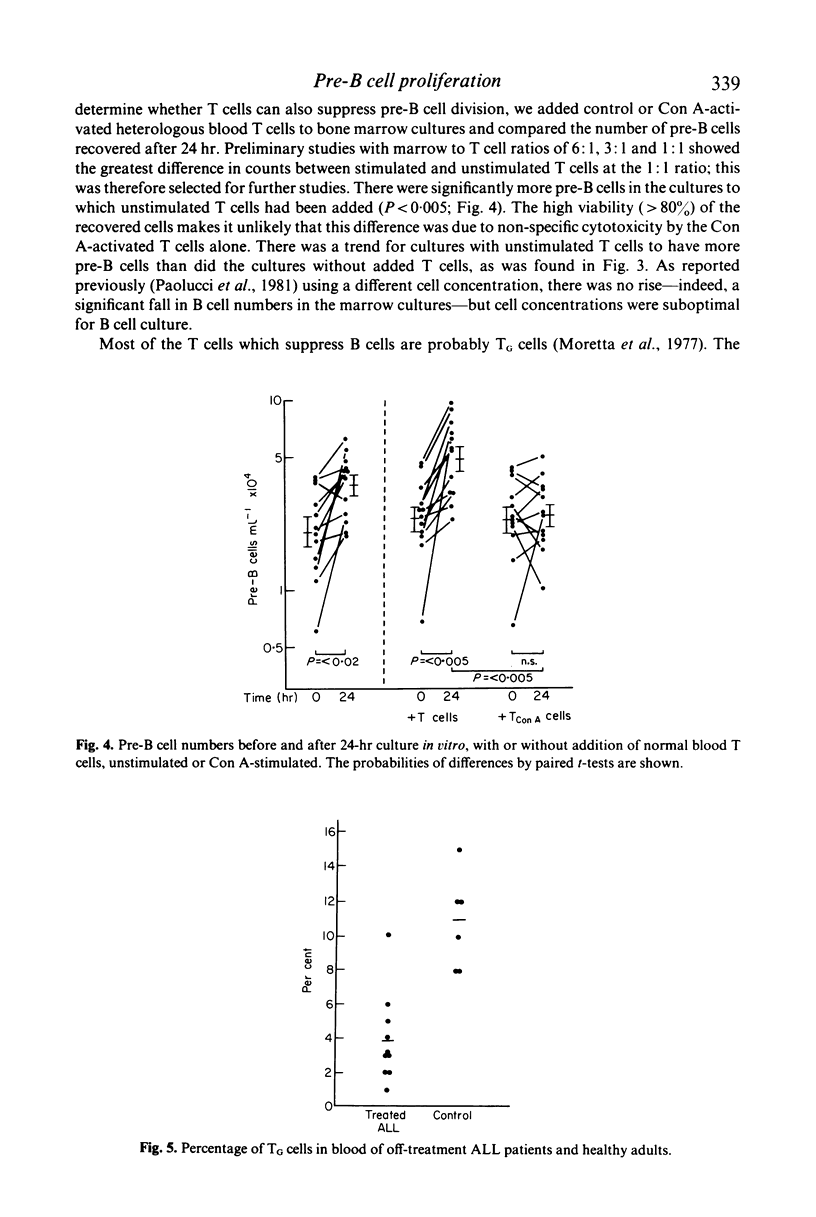
T cells may either increase or decrease in vitro proliferation of marrow pre-B cells from patients with acute lymphatic leukaemia after withdrawal of successful treatment. There is less proliferation when T cells are removed by E rosetting, and repletion of T cells restores proliferation. When additional T cells from the patients were added to the patients' marrows, proliferation was increased more effectively than with T cells from healthy subjects; there was no evidence of an allogeneic effect. In contrast, normal T cells stimulated with concanavalin A suppress proliferation. There was no evidence of differentiation into B cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrows P. D., Kearney J. F., Lawton A. R., Cooper M. D. Pre-B cells: bone marrow persistence in anti-mu-suppressed mice, conversion to B lymphocytes, and recovery after destruction by cyclophosphamide. J Immunol. 1978 May;120(5):1526–1531. [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Layward L., Lydyard P. M., Moretta L., Dagg M., Lawton A. R. Fc-receptor heterogeneity of human suppressor T cells. J Immunol. 1978 Jul;121(1):1–5. [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolucci P., Hayward A. R., Rapson N. T. Pre-B and B cells in children on leukaemia remission maintenance treatment. Clin Exp Immunol. 1979 Aug;37(2):259–266. [PMC free article] [PubMed] [Google Scholar]
- Paolucci P., Rapson N. T., Layward L., Hayward A. R. Growth of pre-B cells in cultures of bone marrow from children with acute lymphoblastic leukaemia and other diseases. Clin Exp Immunol. 1981 Jan;43(1):143–148. [PMC free article] [PubMed] [Google Scholar]
- Trompeter R. S., Layward L., Hayward A. R. Primary and secondary abnormalities of T cell subpopulations. Clin Exp Immunol. 1978 Dec;34(3):388–392. [PMC free article] [PubMed] [Google Scholar]


