Abstract
Cultured rabbit peritoneal macrophages, after stimulation with lipopolysaccharides (LPS), produce a factor that induces normal rabbit articular cartilage cells (chondrocytes) to release collagenase and other neutral proteases in their culture medium. The release of the factor as well as the activation of chondrocytes can be significantly inhibited by paramethasone (10(-6) M). Rabbit peripheral blood monocytes produce this factor in smaller quantities. Activation with LPS does not enhance the release of factor any further by these cells. Lymphocytes have no direct effect on the chondrocytic protease synthesis. Furthermore, conditioned medium of activated lymphocytes failed to stimulate monocytes or macrophages in the absence of LPS. The macrophage medium exhibits mitogenic and phytohaemagglutinin-enhancing activity towards thymocytes of C3H/HeJ mice, but not against species-specific rabbit lymphocytes. The lymphocyte-activating factor, derived from a mouse macrophage cell line, P388D1 cells, or from other sources, was unable to stimulate chondrocytic protease secretion. Such specific induction of chondrocytic proteases by a macrophage-derived factor may have an important role in cartilage destruction in arthritic conditions, where synovium is only marginally involved.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Blyden G., Handschumacher R. E. Purification and properties of human lymphocyte activating factor (LAF). J Immunol. 1977 May;118(5):1631–1638. [PubMed] [Google Scholar]
- Dayer J. M., Bréard J., Chess L., Krane S. M. Participation of monocyte-macrophages and lymphocytes in the production of a factor that stimulates collagenase and prostaglandin release by rheumatoid synovial cells. J Clin Invest. 1979 Nov;64(5):1386–1392. doi: 10.1172/JCI109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Graham R., Russell G., Krane S. M. Collagenase production by rheumatoid synovial cells: stimulation by a human lymphocyte factor. Science. 1977 Jan 14;195(4274):181–183. doi: 10.1126/science.188134. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
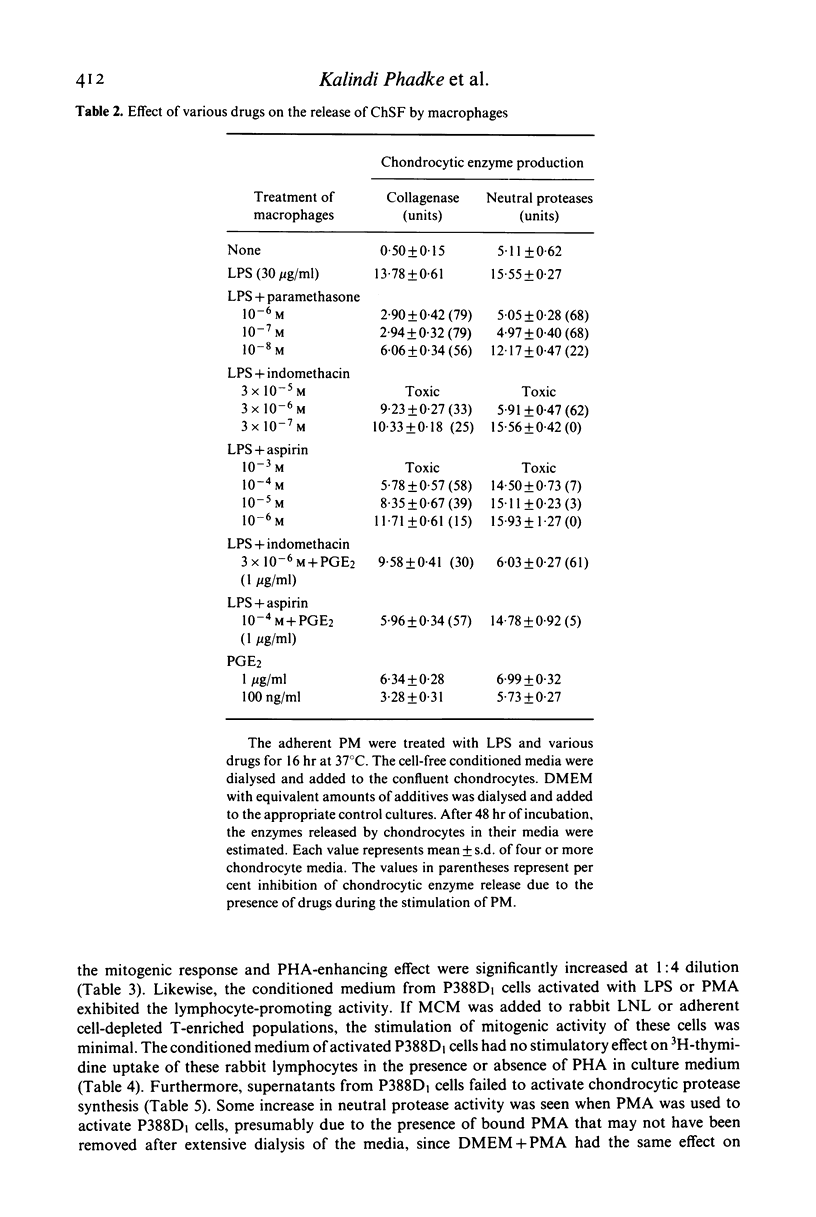
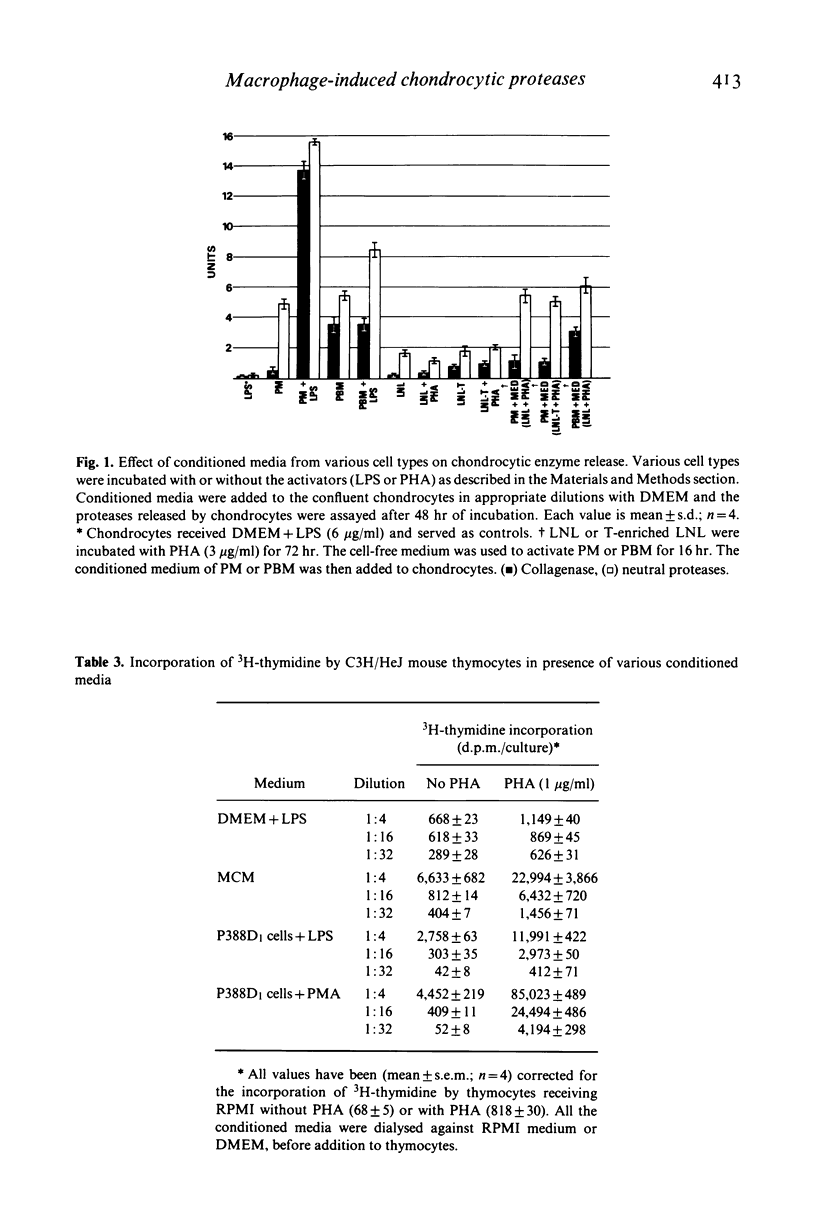
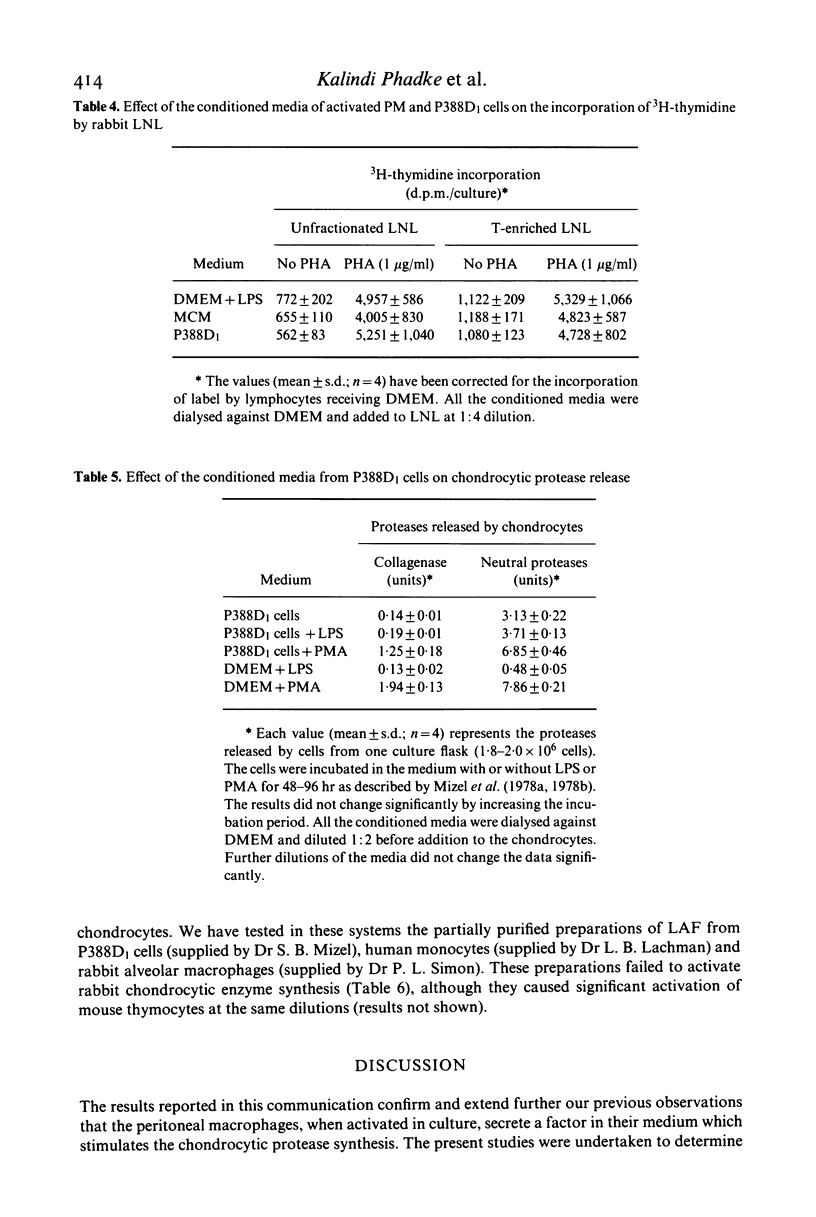
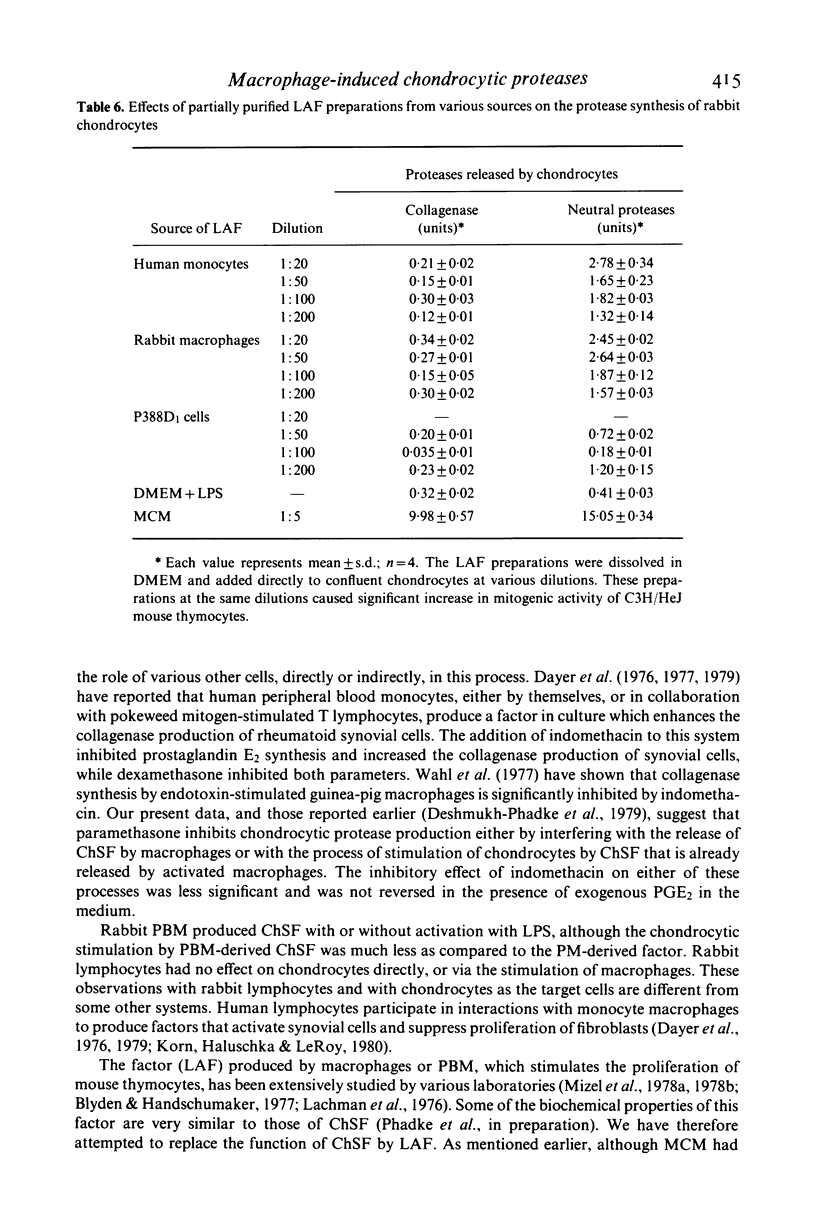
- Deshmukh-Phadke K., Lawrence M., Nanda S. Synthesis of collagenase and neutral proteases by articular chondrocytes: stimulation by a macrophage-derived factor. Biochem Biophys Res Commun. 1978 Nov 14;85(1):490–496. doi: 10.1016/s0006-291x(78)80068-0. [DOI] [PubMed] [Google Scholar]
- Deshmukh-Phadke K., Nanda S., Lee K. Macrophage factor that induces neutral protease secretion by normal rabbit chondrocytes. Studies of some properties and effects on metabolism of chondrocytes. Eur J Biochem. 1980 Feb;104(1):175–180. doi: 10.1111/j.1432-1033.1980.tb04413.x. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (third of three parts). N Engl J Med. 1974 Sep 26;291(13):652–661. doi: 10.1056/NEJM197409262911305. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Rosenstreich D. L., Oppenheim J. J. Phorbol myristic acetate stimulates LAF production by the macrophage cell line, P388D. Cell Immunol. 1978 Sep 15;40(1):230–235. doi: 10.1016/0008-8749(78)90330-1. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke K., Nanda S., Lee K. Release of proteases from cartilage cells as a result of activation by a macrophage factor--effects of some anti-inflammatory drugs. Biochem Pharmacol. 1979 Dec 15;28(24):3671–3673. doi: 10.1016/0006-2952(79)90416-7. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Olsen C. E., Sandberg A. L., Mergenhagen S. E. Prostaglandin regulation of macrophage collagenase production. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4955–4958. doi: 10.1073/pnas.74.11.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]


