Abstract
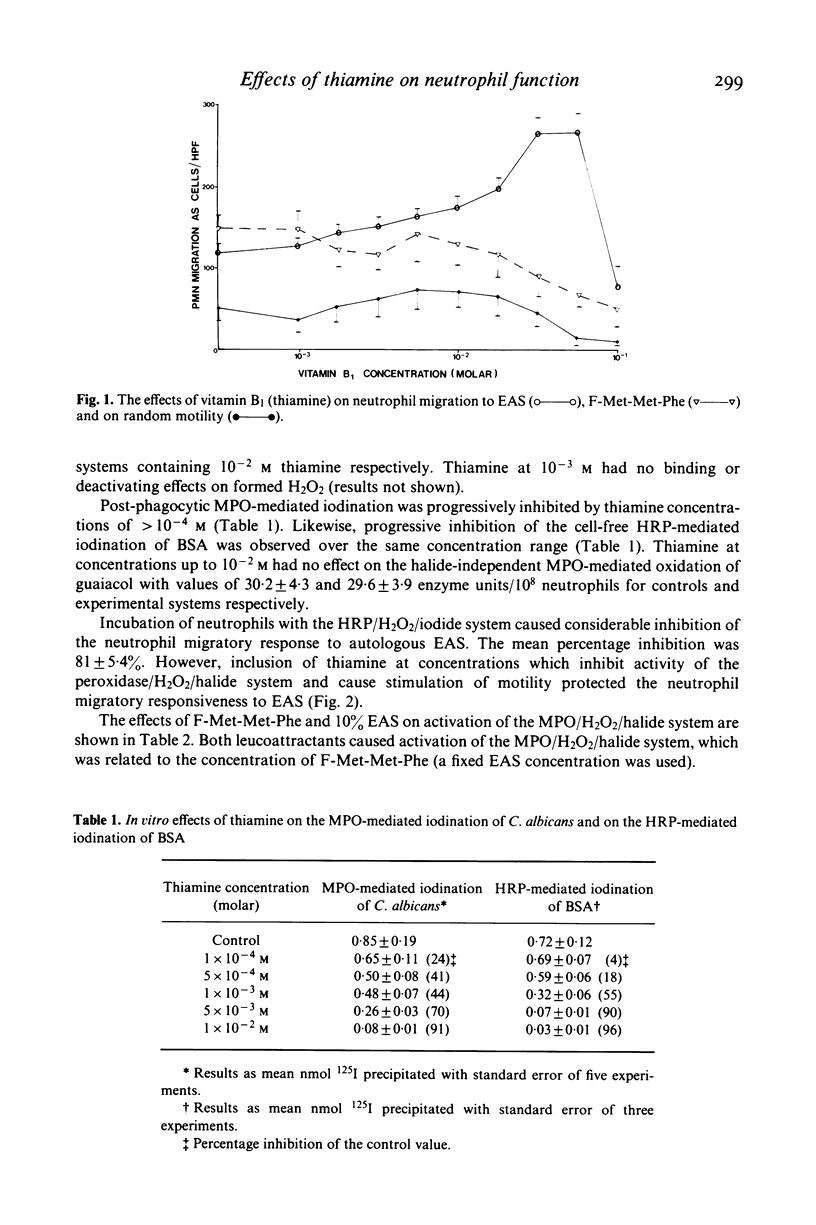
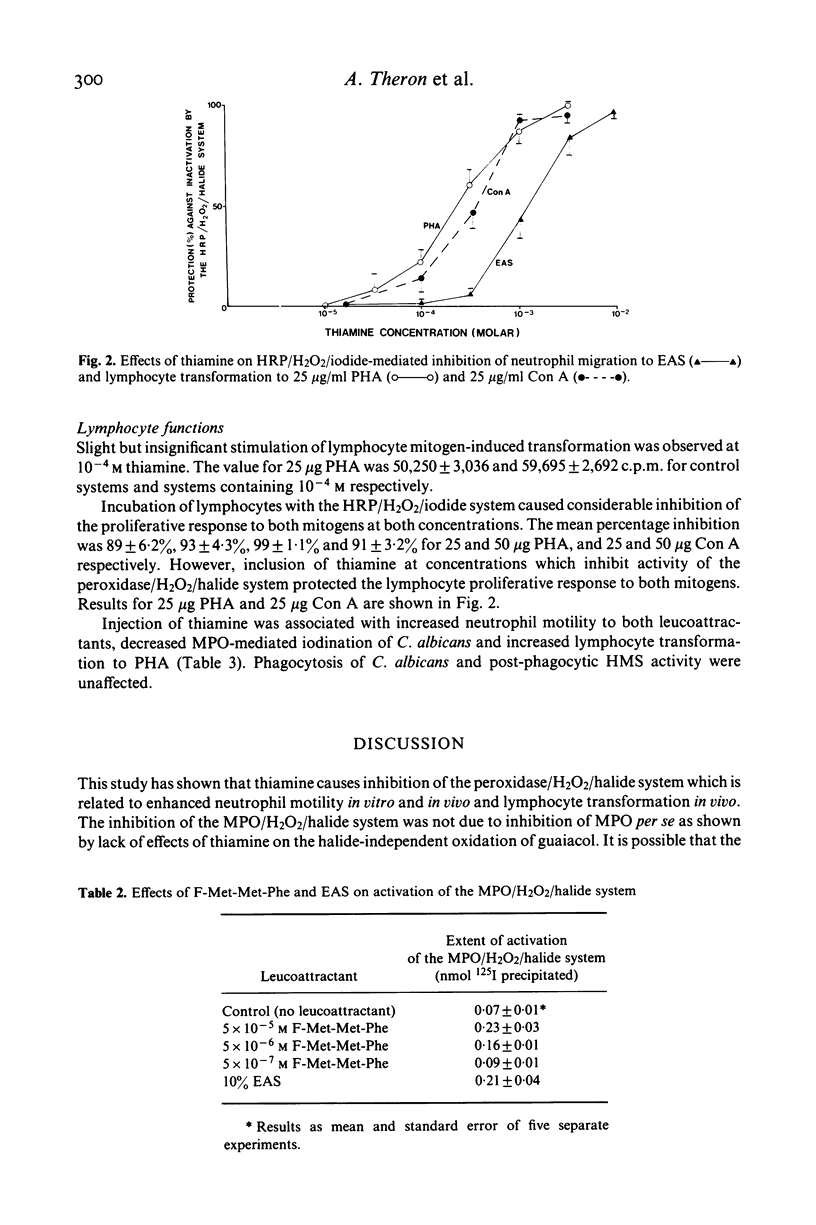
The effects of thiamine on neutrophil functions and mitogen-induced lymphocyte transformation were investigated in vitro and in vivo in adult volunteers following the injection of 50 mg thiamine intramuscularly. Thiamine caused stimulation of neutrophil motility in vitro and in vivo and increased lymphocyte transformation in vivo. Enhancement of these functions was related to inhibition of neutrophil post-phagocytic iodination of Candida albicans by the MPO/H2O2/halide system. The horseradish peroxidase/-H2O2/125 I-mediated iodination of bovine serum albumin was also inhibited by thiamine concentrations which caused increased neutrophil motility. It was found that preincubation of neutrophils and lymphocytes with the horseradish peroxidase/H2O2/halide system caused considerable inhibition of the migratory and proliferative responses respectively. Inclusion of thiamine at concentrations which were found to inhibit the peroxidase/-H2O2/halide system protected the neutrophil migratory and lymphocyte proliferative responses from inactivation by this system. It is suggested that thiamine may cause increased neutrophil migration and lymphocyte transformation by protecting these cells from toxic oxidative products generated by the peroxidase/H2O2/halide system.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Glover A., Rabson A. R. The effect of chemotactic factors and agents which influence neutrophil movement on anaerobic glycolysis and hexose monophosphate shunt activity. Immunology. 1978 Jul;35(1):141–149. [PMC free article] [PubMed] [Google Scholar]
- Anderson R., van Rensburg A. J. The in vitro effects of propranolol and atenolol on neutrophil motility and post-phagocytic metabolic activity. Immunology. 1979 May;37(1):15–24. [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Baehner R. L. Potentiation of polymorphonuclear leukocyte motile functions by 2,3-dihydroxybenzoic acid. J Lab Clin Med. 1978 Nov;92(5):730–736. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. C., Wu M. Y., Rivera-Arcilla J. Inhibition of lymphycyte reactivity in vitro by autologous polymorphonuclear cells (PMN). Cell Immunol. 1979 Dec;48(2):288–295. doi: 10.1016/0008-8749(79)90123-0. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. B., Selvaraj R. J., Sbarra A. J. A sensitive assay method for peroxidases from various sources. J Reticuloendothel Soc. 1978 May;23(5):407–410. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]


