Abstract
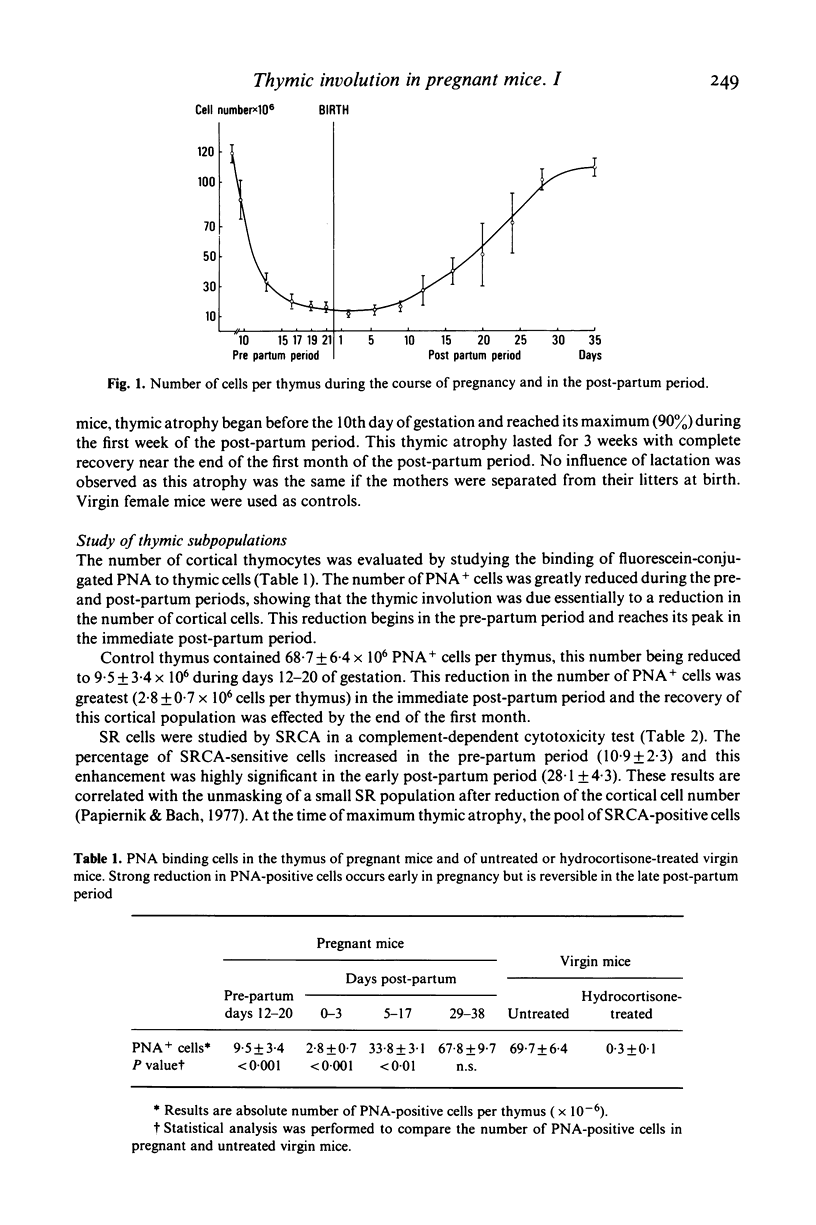
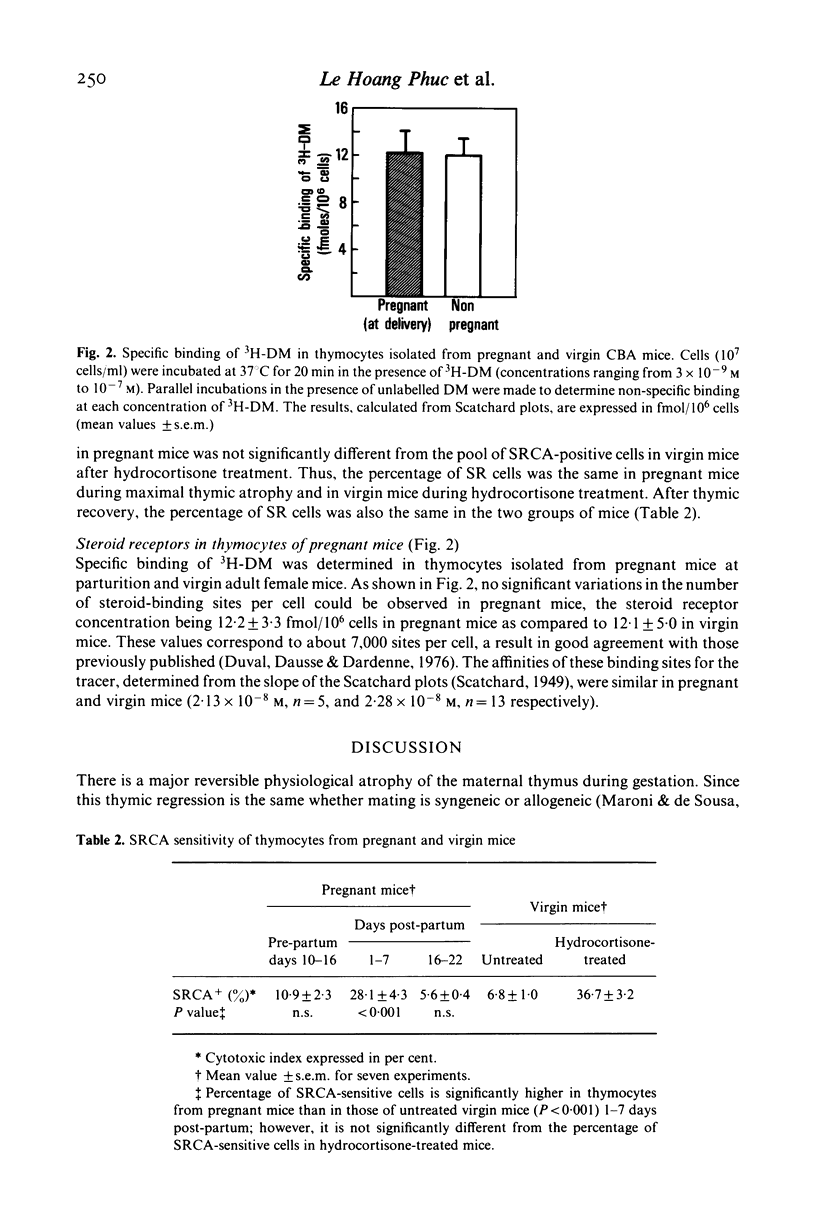
Pregnancy-induced thymic atrophy was studied in mice during the course of syngeneic gestation and the post-partum period. Cortical thymocytes were greatly reduced in number as shown by the binding of fluorescein-labelled PNA. The pool of steroid-resistant (SR) medullary thymocytes appeared unchanged in pregnant mice when studied by means of a specific heteroantiserum (SRCA). Therefore, in pregnant mice, these two surface markers demonstrated that thymic atrophy was linked to steroid-sensitive (SS) cortical cell reduction. The presumed hydrocortisone resistance of the mother's remaining thymocytes is not related to a difference in the number of steroid receptors as determined by 3H-dexamethasone binding.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J. The responsiveness of various maternal mouse lymphocyte populations to mitogenic stimulation in vitro. Cell Immunol. 1978 Nov;41(1):150–156. doi: 10.1016/s0008-8749(78)80034-3. [DOI] [PubMed] [Google Scholar]
- Baines M. G., Pross H. F., Millar K. G. Effects of pregnancy on the maternal lymphoid system in mice. Obstet Gynecol. 1977 Oct;50(4):457–461. [PubMed] [Google Scholar]
- DOUGHERTY T. F., BERLINER M. L., SCHNEEBELI G. L., BERLINER D. L. HORMONAL CONTROL OF LYMPHATIC STRUCTURE AND FUNCTION. Ann N Y Acad Sci. 1964 Feb 28;113:825–843. doi: 10.1111/j.1749-6632.1964.tb40707.x. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY T. F. Effect of hormones on lympatic tissue. Physiol Rev. 1952 Oct;32(4):379–401. doi: 10.1152/physrev.1952.32.4.379. [DOI] [PubMed] [Google Scholar]
- Duval D., Dausse J. P., Dardenne M. Glucocorticoid receptors in corticosensitive and corticoresistant thymocyte subpopulations. I. characterization of glucocorticoid receptors and isolation of a corticoresistant subpopulation. Biochim Biophys Acta. 1976 Nov 18;451(1):82–91. doi: 10.1016/0304-4165(76)90259-2. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Garrett T. J. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972 Nov 1;136(5):1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIDATE M., METCALF D. THE PATTERN OF LYMPHOPOIESIS IN THE MOUSE THYMUS AFTER CORTISONE ADMINISTRATION OR ADRENALECTOMY. Aust J Exp Biol Med Sci. 1963 Dec;41:637–649. doi: 10.1038/icb.1963.53. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Halterman R. H., Leventhal B. G., Perry S., Thompson E. B. Glucocorticoid-binding proteins in human acute lymphoblastic leukemic blast cells. J Clin Invest. 1973 Jul;52(7):1715–1725. doi: 10.1172/JCI107353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Berrih S., Bach J. F. Peanut agglutinin. I. A new tool for studying T lymphocyte subpopulations. J Immunol. 1978 Aug;121(2):438–443. [PubMed] [Google Scholar]
- Maroni E. S., de Sousa M. A. The lymphoid organs during pregnancy in the mouse. A comparison between a syngeneic and an allogeneic mating. Clin Exp Immunol. 1973 Jan;13(1):107–124. [PMC free article] [PubMed] [Google Scholar]
- Millar K. G., Mills P., Baines M. G. A study of the influence of pregnancy on the thymus gland of the mouse. Am J Obstet Gynecol. 1973 Dec 1;117(7):913–918. doi: 10.1016/0002-9378(73)90061-6. [DOI] [PubMed] [Google Scholar]
- Nelson J. H., Jr, Hall J. E., Manuel-Limson G., Freidberg H., O'Brien F. J. Effect of pregnancy on the thymolymphatic system. I. Changes in the intact rat after exogenous HCG, estrogen, and progesterone administration. Am J Obstet Gynecol. 1967 Aug 1;98(7):895–899. [PubMed] [Google Scholar]
- Nelson J. H., Jr, Lu T., Hall J. E., Krown S., Nelson J. M., Fox C. W. The effect of trophoblast on immune state of women. Am J Obstet Gynecol. 1973 Nov 1;117(5):689–699. doi: 10.1016/0002-9378(73)90211-1. [DOI] [PubMed] [Google Scholar]
- PEPPER F. J. The effect of age, pregnancy and lactation on the thymus gland and lymph nodes of the mouse. J Endocrinol. 1961 Jul;22:335–348. doi: 10.1677/joe.0.0220335. [DOI] [PubMed] [Google Scholar]
- Papiernik M., Bach J. F. Thymocyte subpopulations in young and adult mice. II. Study of steroid-resistant populations by means of a specific heteroantiserum. Eur J Immunol. 1977 Nov;7(11):800–803. doi: 10.1002/eji.1830071111. [DOI] [PubMed] [Google Scholar]
- Papiernik M., Laroche L., Bach J. F. Thymocyte subpopulations in young and adult mice. I. Separation by density gradient and steroid treatment. Eur J Immunol. 1977 Nov;7(11):796–799. doi: 10.1002/eji.1830071110. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Rosenau W., Baxter J. D., Rousseau G. G., Tomkins G. M. Mechanism of resistance to steroids: glucocorticoid receptor defect in lymphoma cells. Nat New Biol. 1972 May 3;237(70):20–24. doi: 10.1038/newbio237020a0. [DOI] [PubMed] [Google Scholar]


