Abstract
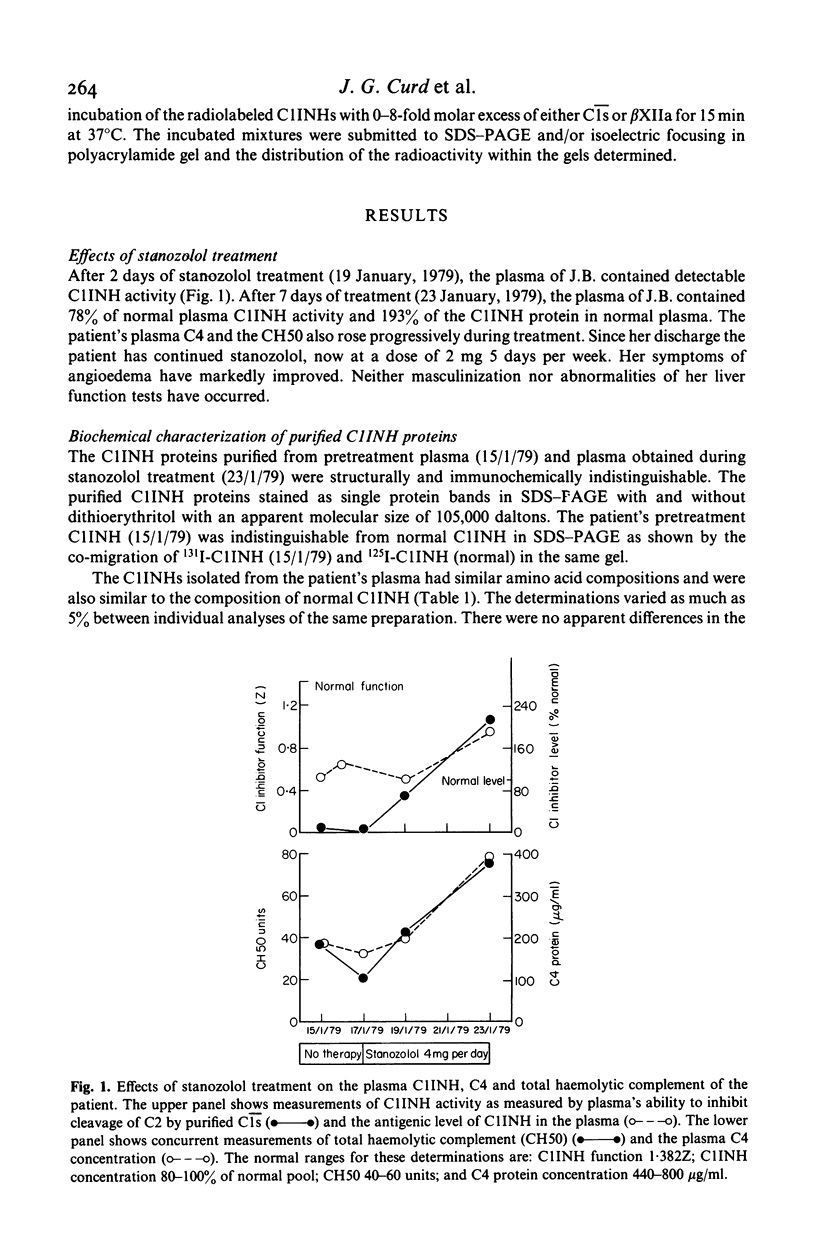
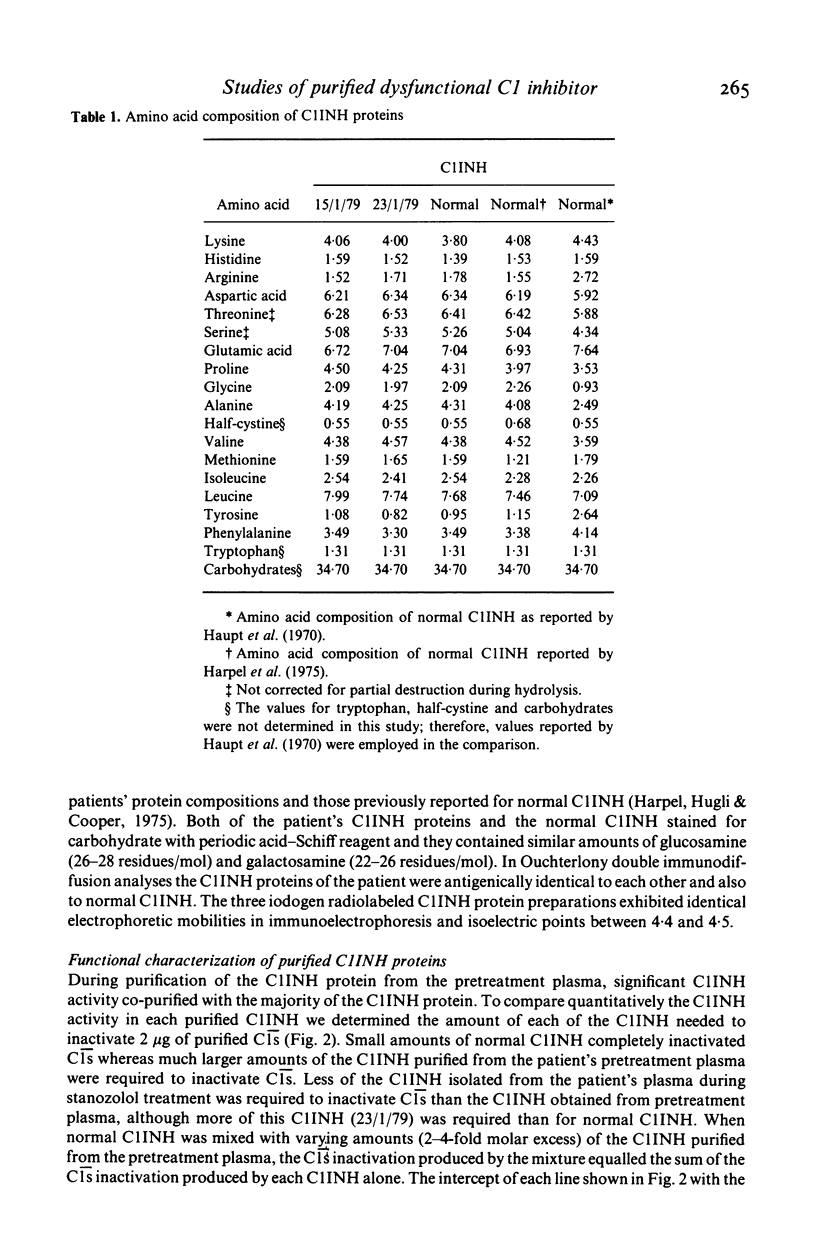
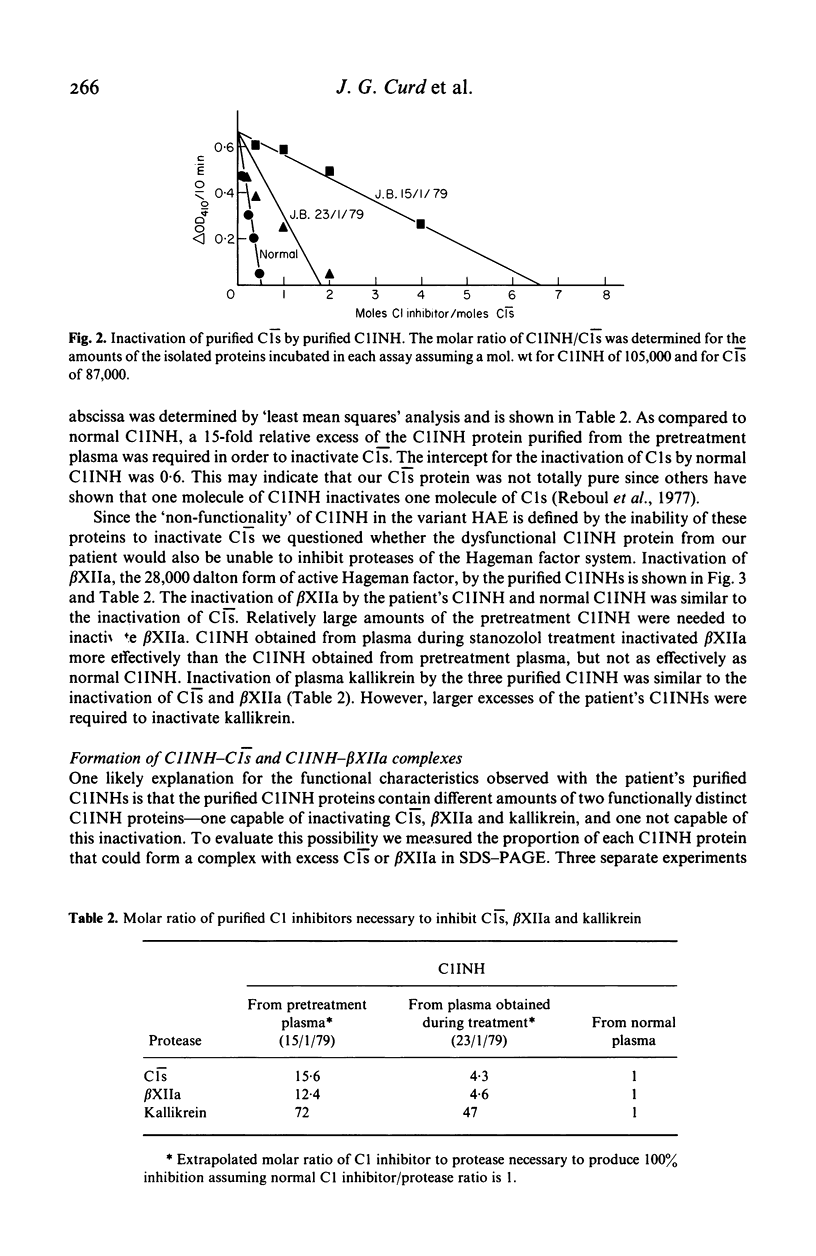
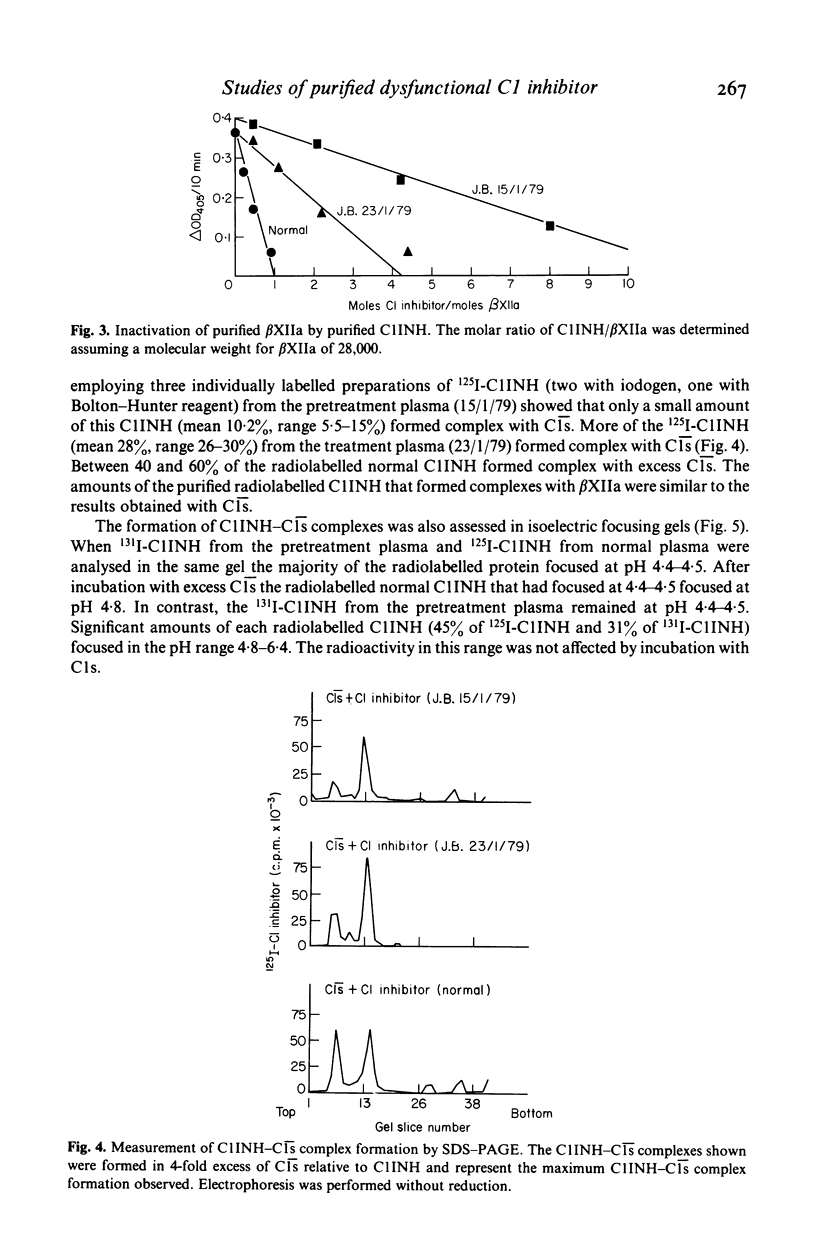
A minority of patients with hereditary angioedema (HAE) have normal concentrations of a dysfunctional C1 inhibitor protein (C1INH) in their plasmas. We purified C1INH from the plasmas of one such patient before and during treatment with the anabolic steroid stanozolol. Both the pretreatment plasma and plasma obtained during stanozolol treatment contained varying amounts of two extremely similar C1INH proteins that were functionally distinct. The pretreatment plasma contained primarily (94%) dysfunctional C1INH that did not inactivate or complex with either purified C1s, activated Hageman factor, or kallikrein and small amounts (6%) of functionally normal C1INH. Stanozolol treatment increased the plasma concentrations of both of these proteins as well as the proportion (23%) of functional C1INH in the plasma. The purified dysfunctional and functional C1INHs had identical or nearly identical molecular sizes, charges, amino acid compositions, and amino sugar contents, and could not be distinguished physicochemically from each other or from normal C1INH. From these studies of purified C1INH proteins we concluded that HAE associated with dysfunctional C1INH is due to a defect at the structural locus for one C1INH gene and that both the dysfunctional C1INH gene and the normal C1INH gene products are present in the plasma of the affected subject. Treatment with stanozolol comparably increased the synthesis of both C1INH proteins. The disproportionate rise in the level of the normal C1INH protein is consistent with the view that it is more rapidly catabolized as a consequence of its interaction with the proteases it inactivates.
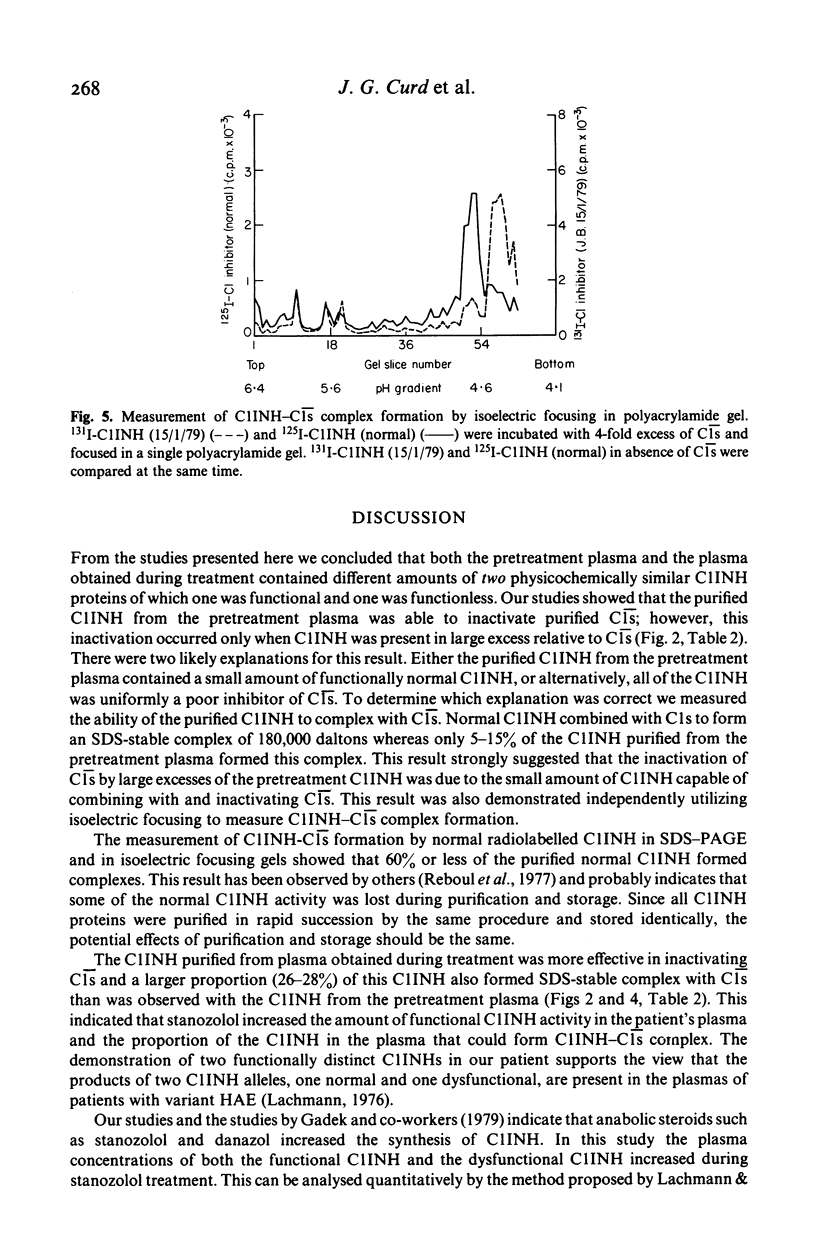
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma B. N., Miles L. A., Beretta G., Griffin J. H. Human plasma prekallikrein. Studies of its activation by activated factor XII and of its inactivation by diisopropyl phosphofluoridate. Biochemistry. 1980 Mar 18;19(6):1151–1160. doi: 10.1021/bi00547a018. [DOI] [PubMed] [Google Scholar]
- Colten H. R. Biosynthesis of complement. Adv Immunol. 1976;22:67–118. doi: 10.1016/s0065-2776(08)60548-9. [DOI] [PubMed] [Google Scholar]
- DONALDSON V. H., EVANS R. R. A BIOCHEMICAL ABNORMALITY IN HEREDIATRY ANGIONEUROTIC EDEMA: ABSENCE OF SERUM INHIBITOR OF C' 1-ESTERASE. Am J Med. 1963 Jul;35:37–44. doi: 10.1016/0002-9343(63)90162-1. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Forbes C. D., Pensky J., Ratnoff O. D. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J Lab Clin Med. 1970 Nov;76(5):809–815. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Frank M. M., Gelfand J. A., Atkinson J. P. Hereditary angioedema: the clinical syndrome and its management. Ann Intern Med. 1976 May;84(5):580–593. doi: 10.7326/0003-4819-84-5-580. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Hosea S. W., Gelfand J. A., Frank M. M. Response of variant hereditary angioedema phenotypes to danazol therapy. Genetic implications. J Clin Invest. 1979 Jul;64(1):280–286. doi: 10.1172/JCI109449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand J. A., Sherins R. J., Alling D. W., Frank M. M. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med. 1976 Dec 23;295(26):1444–1448. doi: 10.1056/NEJM197612232952602. [DOI] [PubMed] [Google Scholar]
- Gigli I., Mason J. W., Colman R. W., Austen K. F. Interaction of plasma kallikrein with the C1 inhibitor. J Immunol. 1970 Mar;104(3):574–581. [PubMed] [Google Scholar]
- Gigli I., Ruddy S., Austen K. F. The stoichiometric measurement of the serum inhibition of the first component of complement by the inhibition of immune hemolysis. J Immunol. 1968 Jun;100(6):1154–1164. [PubMed] [Google Scholar]
- Harpel P. C., Cooper N. R. Studies on human plasma C1 inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. 1975 Mar;55(3):593–604. doi: 10.1172/JCI107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C., Hugli T. E., Cooper N. R. Studies on human plasma C1 inactivator-enzyme interactions. II. Structural features of an abnormal C1 inactivator from a kindred with hereditary angioneurotic edema. J Clin Invest. 1975 Mar;55(3):605–611. doi: 10.1172/JCI107968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt H., Heimburger N., Kranz T., Schwick H. G. Ein Beitrag zur Isolierung und Charakterisierung des Cl-Inaktivators aus Humanplasma. Eur J Biochem. 1970 Dec;17(2):254–261. doi: 10.1111/j.1432-1033.1970.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Houston L. L. Amino acid analysis of stained bands from polyacrylamide gels. Anal Biochem. 1971 Nov;44(1):81–88. doi: 10.1016/0003-2697(71)90348-4. [DOI] [PubMed] [Google Scholar]
- LEVY L. R., LEPOW I. H. Assay and properties of serum inhibitor of C'l-esterase. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:608–611. doi: 10.3181/00379727-101-25034. [DOI] [PubMed] [Google Scholar]
- Laurell A. B., Mårtensson U. C1 inactivator protein complexed with albumin in plasma from a patient with angioneurotic edema. Eur J Immunol. 1971 Apr;1(2):146–149. doi: 10.1002/eji.1830010215. [DOI] [PubMed] [Google Scholar]
- ROSEN F. S., PENSKY J., DONALDSON V., CHARACHE P. HEREDITARY ANGIONEUROTIC EDEMA: TWO GENETIC VARIANTS. Science. 1965 May 14;148(3672):957–958. doi: 10.1126/science.148.3672.957. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D., Pensky J., Ogston D., Naff G. B. The inhibition of plasmin, plasma kallikrein, plasma permeability factor, and the C'1r subcomponent of the first component of complement by serum C'1 esterase inhibitor. J Exp Med. 1969 Feb 1;129(2):315–331. doi: 10.1084/jem.129.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul A., Arlaud G. J., Sim R. B., Colomb M. G. A simplified procedure for the purification of C1-inactivator from human plasma. Interaction with complement subcomponents C1r and C1s. FEBS Lett. 1977 Jul 1;79(1):45–50. doi: 10.1016/0014-5793(77)80347-5. [DOI] [PubMed] [Google Scholar]
- Rosen F. S., Alper C. A., Pensky J., Klemperer M. R., Donaldson V. H. Genetically determined heterogeneity of the C1 esterase inhibitor in patients with hereditary angioneurotic edema. J Clin Invest. 1971 Oct;50(10):2143–2149. doi: 10.1172/JCI106708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. D., Kaplan A. P., Austen K. F. Inhibition by C1INH of Hagemann factor fragment activation of coagulation, fibrinolysis, and kinin generation. J Clin Invest. 1973 Jun;52(6):1402–1409. doi: 10.1172/JCI107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Reboul A., Arlaud G. J., Villiers C. L., Colomb M. G. Interaction of 125I-labelled complement subcomponents C-1r and C-1s with protease inhibitors in plasma. FEBS Lett. 1979 Jan 1;97(1):111–115. doi: 10.1016/0014-5793(79)80063-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Active disassembly of the first complement component, C-1, by C-1 inactivator. J Immunol. 1979 Aug;123(2):788–792. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Modulation of the antigenicity of C1r and C1s by C1 inactivator. J Immunol. 1978 Dec;121(6):2148–2152. [PubMed] [Google Scholar]


