Abstract
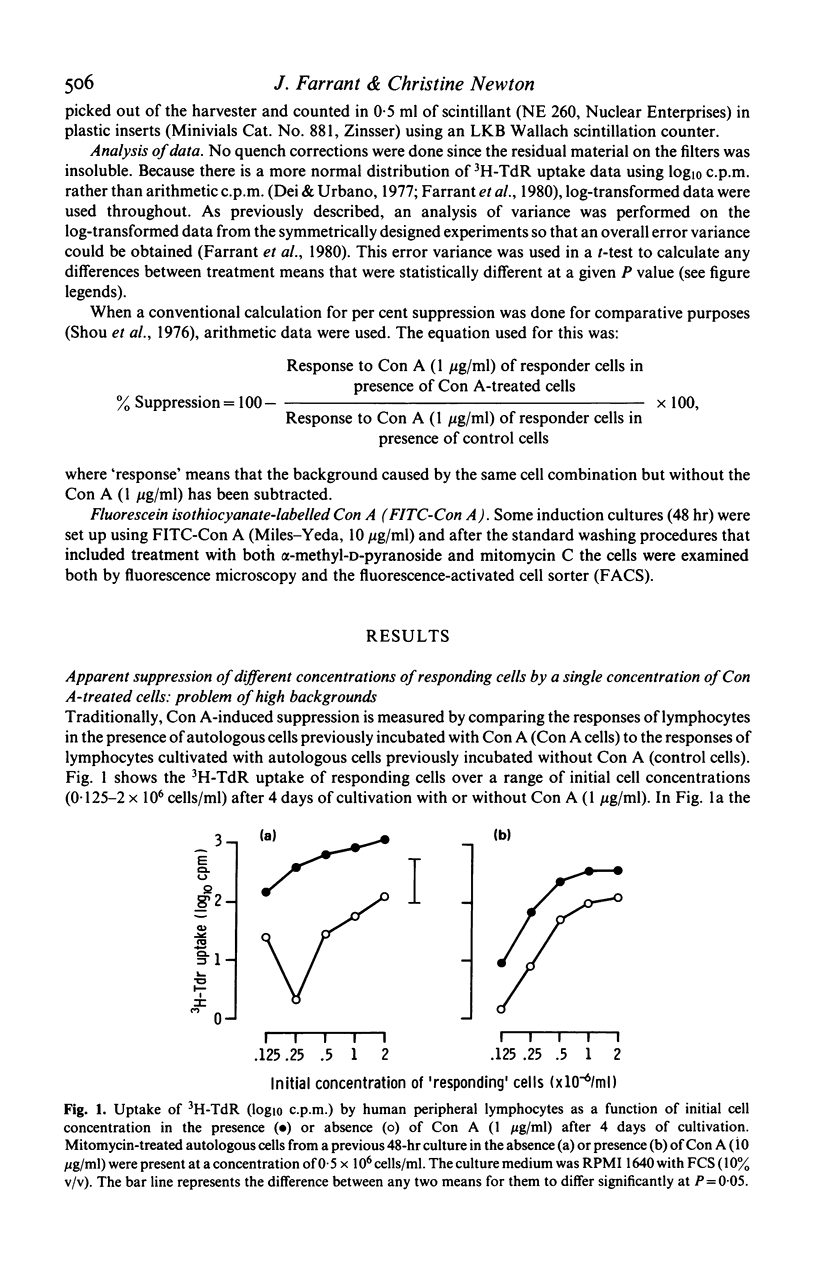
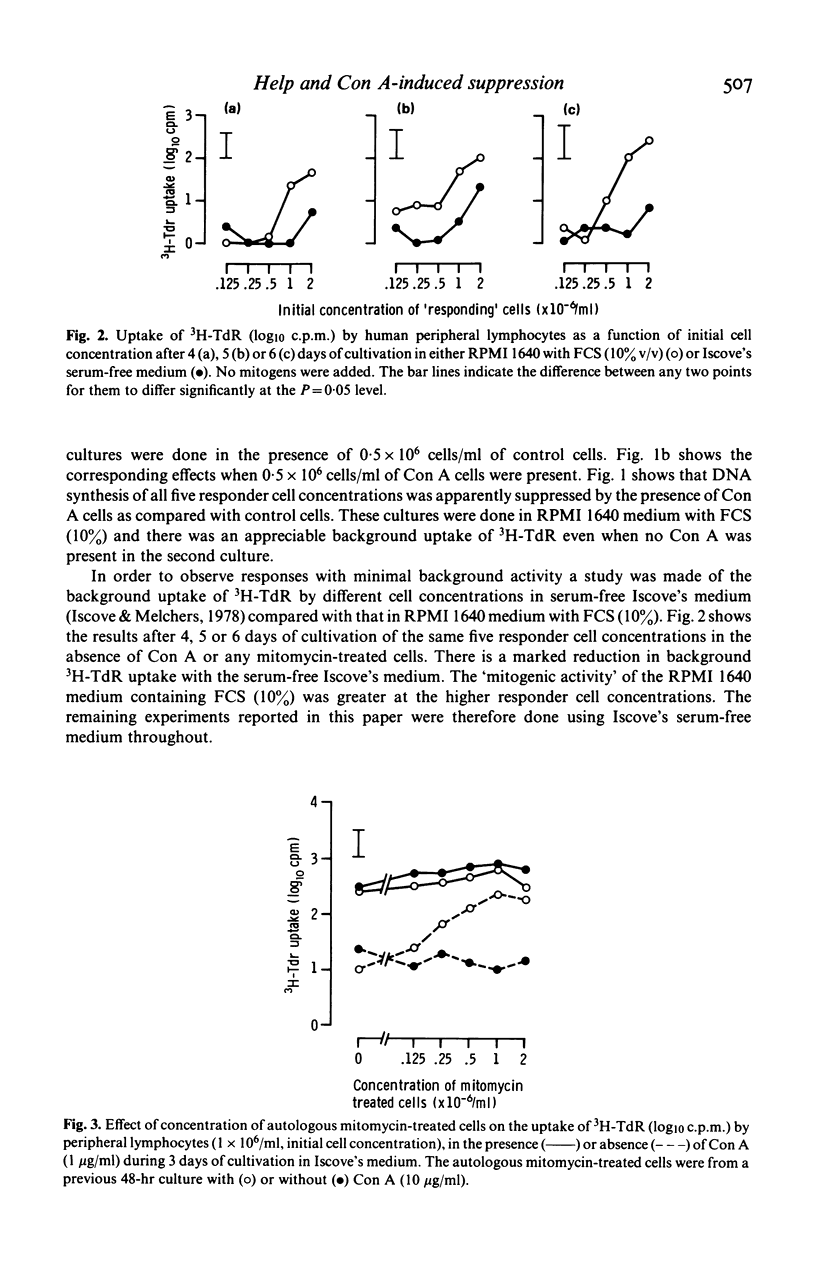
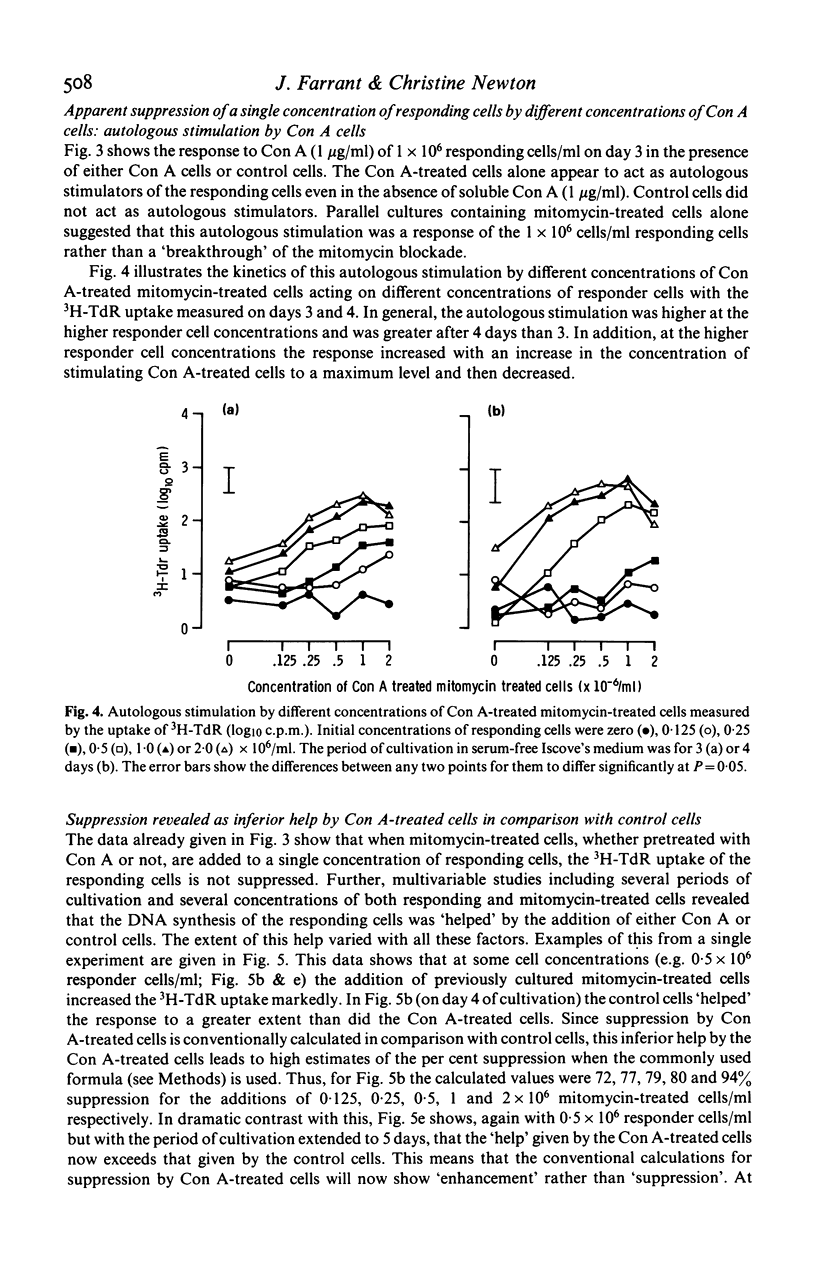
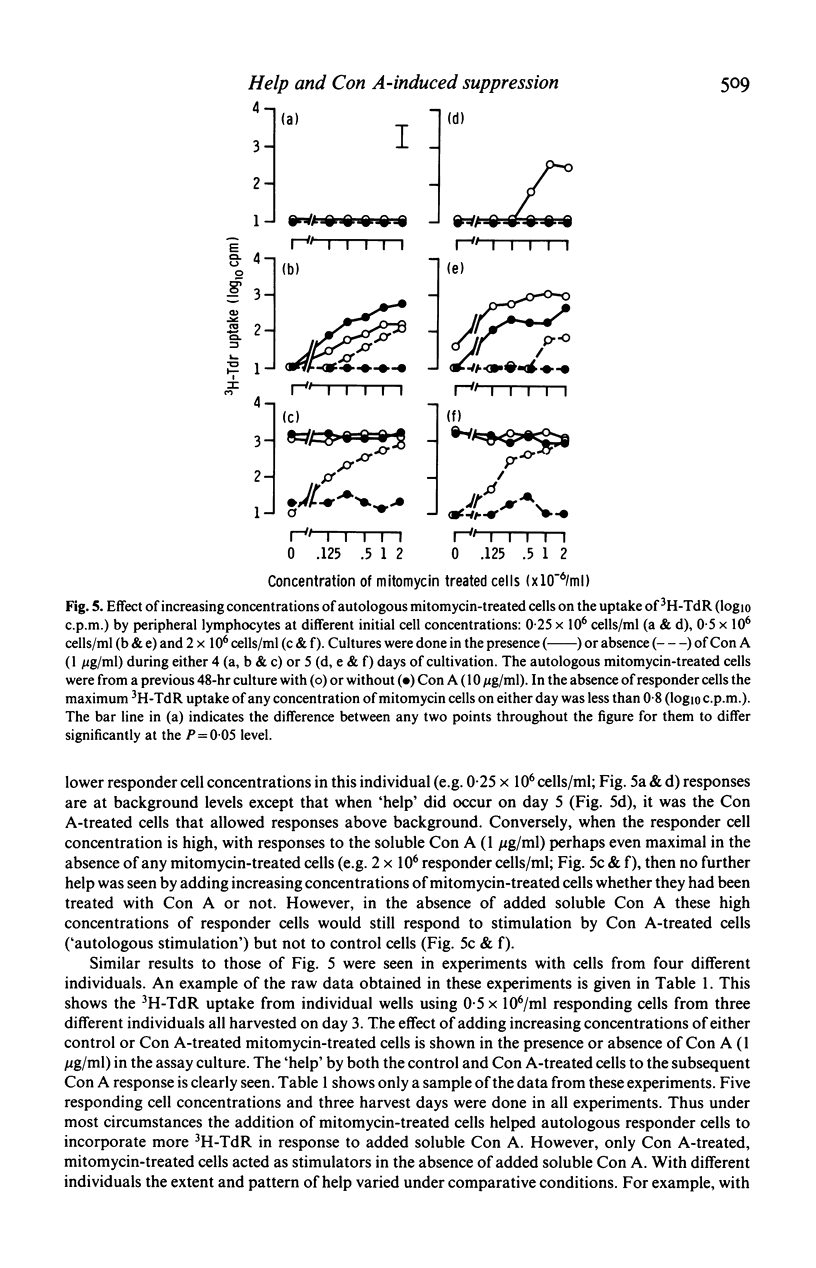
Concanavalin A (Con A) induced suppression of human peripheral lymphocytes has been studied in vitro at different cell concentrations using a 20 microliters inverted Terasaki plate culture system in which the uptake of 3H-thymidine was measured. Conventional assessment of suppression relates the response of cells to Con A in the presence of Con A-pretreated autologous cells, with the response of cells preincubated without Con A (control cells). The level of suppression so calculated was affected both by serum-related high background counts and by autologous stimulation of responding cells by mitomycin-treated Con A-pretreated cells. The high background was largely removed by the use of serum-free Iscove's medium. The data involving different cell concentrations and periods of cultivation indicate that the assessment of suppression is inadequate. Both Con A-pretreated and control cells are shown to 'help' the response of autologous responder cells to added soluble Con A. When the help by Con A-treated cells is less marked than that by control cells, apparent suppression is seen since the control cell provide the conventional baseline from which suppression is judged. After longer periods of cultivation the Con A-pretreated cells increased responses more than did the control cells leading to apparent enhancement.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnason B. G., Antel J. Suppressor cell function in multiple sclerosis. Ann Immunol (Paris) 1978 Feb-Mar;129(2-3):159–170. [PubMed] [Google Scholar]
- De Gast G. C., The T. H., Ponds E., Kallenberg C. Suppression of DNA synthesis by Con A-activated human lymphocytes: Stimulation by con A bound to non-T cells unless removed after activation. Clin Exp Immunol. 1977 Dec;30(3):457–464. [PMC free article] [PubMed] [Google Scholar]
- Dei R., Urbano P. Biometrical implications of factorial experiments for the study of lymphocyte mitogenic response. J Immunol Methods. 1977;15(2):169–181. doi: 10.1016/0022-1759(77)90028-x. [DOI] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. I. Characterization of the inhibitory cell activity. J Exp Med. 1972 Dec 1;136(6):1445–1460. doi: 10.1084/jem.136.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Johnson C., Desaules M. Behaviour of human immunoregulatory cells in culture. I. Variables requiring consideration for clinical studies. Clin Exp Immunol. 1979 Dec;38(3):499–513. [PMC free article] [PubMed] [Google Scholar]
- Farrant J., Clark J. C., Lee H., Knight S. C., O'Brien J. Conditions for measuring DNA synthesis in PHA stimulated human lymphocytes in 20 microliters hanging drops with various cell concentrations and periods of culture. J Immunol Methods. 1980;33(4):301–312. doi: 10.1016/0022-1759(80)90001-0. [DOI] [PubMed] [Google Scholar]
- Farrant J., Knight S. C. Help and suppression by lymphoid cells as a function of cellular concentration. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3507–3510. doi: 10.1073/pnas.76.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighery C., Whelan C. A., Weir D. G., Greally J. F. In vitro studies of suppressor cell function in human peripheral blood mononuclear cells. Clin Exp Immunol. 1978 Jun;32(3):459–465. [PMC free article] [PubMed] [Google Scholar]
- Fernandez L. A., Macsween J. M. Generation of suppressor cells by concanavalin A: a new perspective. J Immunol. 1980 Jul;125(1):267–269. [PubMed] [Google Scholar]
- Fineman S. M., Mudawwar F. B., Geha R. S. Characteristics and mechanisms of action of the concanavalin A-activated suppressor cell in man. Cell Immunol. 1979 Jun;45(1):120–132. doi: 10.1016/0008-8749(79)90367-8. [DOI] [PubMed] [Google Scholar]
- Hallgren H. M., Yunis E. J. Suppressor lymphocytes in young and aged humans. J Immunol. 1977 Jun;118(6):2004–2008. [PubMed] [Google Scholar]
- Hubert C., Delespesse G., Govaerts A. Concanavalin A-activated suppressor cells in normal human peripheral blood lymphocytes. Clin Exp Immunol. 1976 Oct;26(1):95–98. [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg C. G., de Gast G. C., The T. H. Suppression of DNA synthesis by con A-activated human lymphocytes: role of monocytes in con A-induced suppression. Clin Exp Immunol. 1980 Sep;41(3):583–590. [PMC free article] [PubMed] [Google Scholar]
- O'Brien J., Knight S., Quick N. A., Moore E. H., Platt A. S. A simple technique for harvesting lymphocytes cultured in Terasaki plates. J Immunol Methods. 1979;27(3):219–223. doi: 10.1016/0022-1759(79)90219-9. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Con A-inducible suppression of MLC: evidence for mediation by the TH2 + T cell subset in man. J Immunol. 1979 Apr;122(4):1335–1341. [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Shand F. L., Orme I. M., Ivanyi J. The induction of suppressor T cells by concanavalin A is independent of cellular proliferation and protein synthesis. Scand J Immunol. 1980;12(3):223–231. doi: 10.1111/j.1365-3083.1980.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]


