Abstract
The TBP-like protein (TLP/TRF2/TLF), which belongs to the TBP family of proteins, is present in all metazoan organisms. Although the human TLP has been reported to interfere with transcription from TATA-containing promoters, the transcription activation potential of TLP in higher animals is obscure. We previously demonstrated that artificially promoter-recruited TLP behaves like an unconventional transcriptional activator. In this study, we investigated the effects of TLP on TATA-less promoters of mouse and human terminal deoxynucleotidyl transferase (TdT) genes by transient reporter assays. As expected, TLP repressed both basal and activator-augmented transcription from the TATA-containing adenovirus major late promoter (MLP) and E1B promoter. On the other hand, however, TLP significantly stimulated both basal and activated transcription from TdT promoters. We investigated the strength of the promoters in chicken DT40 cells that lack the TLP gene. The MLP showed higher activity but the TdT promoter showed lower activity in TLP-null cells than in the wild-type cells. Moreover, ectopic expression of mouse TLP in the TLP-null cells considerably stimulated the TdT promoter. Insertion of a TATA element upstream from the TdT core promoter resulted in a loss of TLP-mediated activation. The mouse TLP was demonstrated to bind specifically to TFIIA with greater strength than TBP. We constructed mutated TLPs having amino acid substitutions that impair TFIIA binding. A representative TLP mutant lacking TFIIA-binding ability could not stimulate transcription from the TdT promoter, whereas that mutation suppressed TLP-mediated transcription repression of TATA promoters. The results of the present study suggest that the vertebrate TLP potentiates exogenous TATA-less promoters and that TFIIA plays an important role in the TLP function.
INTRODUCTION
Initiation of eukaryotic transcription is governed by multiple general transcription factors (GTFs) that assemble to a promoter and form a functional preinitiation complex (1–3). TATA-binding protein (TBP) is one of the GTFs and is used for all classes of RNA polymerases (RNAPs) (4). In RNAPII transcription, TBP forms transcription factor IID (TFIID) with TBP-associated factors and binds to the TATA sequence. Transcription factor IIA (TFIIA) promotes this process. Although the mechanism of the initiation of transcription for TATA-less promoters is still obscure, TBP is known to play a central role in the expression of TATA-containing genes.
It is known that higher eukaryotes have proteins that are structurally similar to TBP. At first, TBP-related factor 1 (TRF1) was identified in Drosophila (5). TRF1 resembles the classical TBP and has both TATA-binding and transcriptional activation ability (6). TRF1 works for a development-related RNAPII gene as well as an RNAPIII gene (7,8). Later, another TBP family protein, TBP-like protein (TLP), was identified in the metazoan organisms (9–11). This protein has also been called TLF (TBP-like factor) (12,13) and TRF2 (14,15). TLP is much more distantly related to TBP than is TRF1 in several aspects. The mouse TLP (mTLP), which is only 40% identical to the mouse TBP (mTBP), does not bind to the TATA sequence and does not activate in vitro transcription (10). Although the mechanistic basis of TLP function is not known, results of gene disruption studies on Caenorhabditis elegans and Xenopus TLPs have suggested that TLP is involved in early development (13,16). TLP was also shown to be involved in spermiogenesis of mice (17,18).
A higher order structure of TLP like TBP, and several findings regarding TLP have led to the speculation that TLP is involved in transcriptional activation (14,19,20). In vivo studies have demonstrated that TLP affects the expression of several cellular genes (13,16–18). Other studies have shown that human TLP works as a passive transcriptional repressor for TATA-containing promoters (15,21). This repressive effect has been explained by the TFIIA titration-out model. In our previous work, TLP was demonstrated to stimulate basal transcription when it was artificially recruited to a promoter (22). However, participation of TLP in transcriptional activation in a natural situation is still obscure. In this study, we found that TLP significantly stimulates transcription from core promoters of TATA-less terminal deoxynucleotidyl transferase (TdT) promoters in a transient reporter assay and that TFIIA plays a role in this modulation.
MATERIALS AND METHODS
Plasmids and DNA constructs
We constructed several effector plasmids derived from pCAG-GS (23) to express transcription factors under the control of the chicken β-actin promoter. FLAG and oligo-histidine (His) tags were joined beforehand at the N-termini of expressed proteins. For example, pTLP and pTBP are expression plasmids for the mTLP (10) and mTBP (24), respectively. A TLP mutant, A32E/L33A, which has mutations at L33 and A32, was constructed from pTLP. This mutant has been confirmed not to associate with TFIIA (T.Nakadai et al., manuscript in preparation). Core promoters of adenovirus major late promoter (MLP) (–35 to +5) (25), adenovirus E1b TATA (TATATAA)-containing basic promoter, mouse TdT (mTdT) (–10 to +30) (26) and human TdT (hTdT) (–30 to +30) were used as reporters. Promoter fragments of MLP, mTdT and hTdT were inserted into the 5×(Gal4-binding site)-carrying firefly luciferase vector (pG5-luc) (Promega) to construct pMLP, pmTdT and phTdT, respectively. The E1b TATA-carrying basic promoter, designated here as Basic, also has a 5×Gal4-binding site. This plasmid is the same as pFR-luc (Stratagene).
pBIND (Promega), which includes a Gal4 DNA-binding domain (from 1 to 147), was fused to each effector protein in this study. pBIND-derived GAL4-VP16, GAL4-TBP and GAL4-TLP contain a VP16 activation domain, mTBP and mTLP, respectively. GAL4-CHOP, GAL4-cJUN and GAL4-CREB are identical to pFA2-cJun, pFA2-CHOP and pFA2-CREB (Stratagene), respectively. We also constructed pMLP-derived pML/Td, which has an MLP TATA-containing DNA fragment (–35 to –10) and mTdT promoter (–10 to +30).
Culture of mammalian cells, transfection and luciferase assay
HeLa cells were used as DNA recipients in most of the assays. The cells were grown in DMEM containing 10% fetal calf serum. In the standard transient luciferase assay, HeLa cells were inoculated into 24-well plates (Falcon) at 2 × 104 cells/well. Cells were transfected with DNA (100 ng each) by Lipofectamine Plus (Gibco) and harvested at 20 h after transfection, and proteins were extracted by the standard method (Promega). The luciferase assay was carried out using a Dual-Luciferase Reporter Assay System (Promega) with 5 µl of cell extracts in 25 µl of the reaction system. Generally, 100 ng of each DNA was used for one transfection assay. Enzyme activities were measured using a luminometer and normalized to protein concentration.
Mammalian two-hybrid assay
HeLa cells were simultaneously transfected with pMLP and two kinds of hybrid proteins: ACT-TLP and Gal4 DNA-binding domain-fused test proteins (e.g. GAL4-TFIIA for the α/β subunit of human TFIIA) (27). ACT-TLP is a CMV enhancer/promoter-driven fusion protein constructed from VP16 activation domain-carrying pACT and TLP sequences. Other test proteins included human TFIIB (GAL4-TFIIB), mTBP (GAL4-TBP) (24), RPB6 of rat RNAPII (GAL4-RPB6) (28), c-Jun (GAL4-cJUN; Stratagene) and CREB (GAL4-CREB; Stratagene). Mutated ACT-TLP proteins, including ACT-TLP/A32E and ACT-TLP/I39R for TFIIA-binding, ACT-TLP/E133R for TFIIB-binding and ACT-TLP/K53E, were designated as described above. ACT-TBP contains the mTBP sequence instead of TLP. Cells were transfected with 100 ng of each DNA and cultured for 24 h. The luciferase activity was determined as described above.
FLAG pull-down assay
Overexpression of FLAG- and His-tagged mTLP proteins in Escherichia coli and purification of the proteins were described previously (10). Purified TLP (5 ng) was adsorbed to M2 agarose (Kodak) in a B buffer containing 20 mM Tris–HCl (pH 8.0), 5 mM MgCl2, 10% glycerol, 0.1% NP40 and 200 mM KCl. The recombinant human TFIIA(α/β) was expressed in E.coli (29). The denatured protein was purified by SDS–PAGE then denatured as previously described (29). The resin was incubated with purified human TFIIA(α/β) at room temperature for 1 h and washed with B buffer. Proteins were eluted by boiling for 2 min. The eluates were subjected to 10% SDS–PAGE and then to western blotting using an anti-TFIIA antibody (29) as previously described (10). Specific signals were revealed by the alkaline phosphatase procedure.
Detection of exogenous TLP
To examine ectopicaly expressed TLPs, HeLa cells transfected with hemagglutinin (HA)-TLP (see above) were cultured for 20 h and whole cell extracts were prepared (10). Cell extracts (10 µg) were separated by 10% SDS–PAGE and subjected to western blotting using anti-TLP (10) and anti-HA antibodies (Clontech).
Chicken DT40 cells
Chicken DT40 cells (30) were maintained as a suspension culture in RPMI 1640 (Gibco) containing 10% fetal calf serum and 1% chicken serum (JRM Biosciences) at 39.5°C. The chicken TLP gene was mapped on chromosome 3 (11). Procedures for disruption of the TLP gene of DT40 cells will be described in detail in another paper (M.Shimada et al., manuscript in preparation). Briefly, the third exons of the chicken TLP gene in both alleles were interrupted by drug-resistant genes, and multiple knock-out cell lines were established. In this study, two independent lines of TLP-null cells (P3 and P17) were used. For luciferase assays, cells (2 × 104) were suspended in 250 µl of Lipofectamine reagent (Gibco) and transfected with DNAs.
RESULTS
TLP stimulates TdT promoters that lack a canonical TATA element
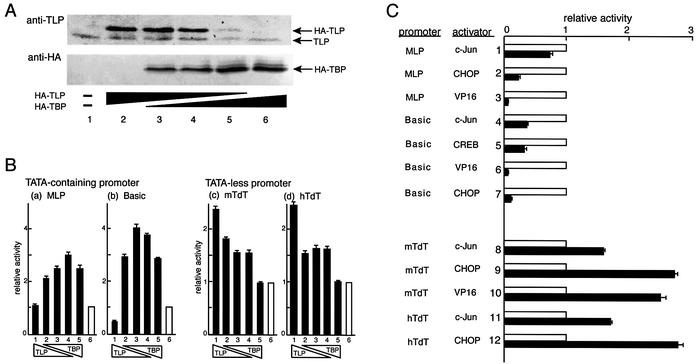
To determine the effect of TLP on in vitro transcription, HeLa cells were transfected together with various doses of the mTLP expression vector (pTLP) as an effector and the MLP-directed luciferase vector (pMLP) as a reporter. A TBP expression plasmid was co-transfected to adjust the amounts of transfected DNAs. Exogenous TLP and TBP were overexpressed well and persisted in cells (Fig. 1A). Although transcription from the core promoter of MLP was not affected by the ectopic TLP expression (Fig. 1Ba, lane 1), another TATA-carrying promoter, Basic, was repressed by 50% when TLP alone was overexpressed (Fig. 1Bb, lane 1), as previously reported (15,21). For both promoters, transfection of decreased amounts of TLP plasmid accompanied by relatively increased amounts of the TBP plasmid stimulated transcription, although the degree of stimulation was slightly decreased when TBP alone was much more overexpressed (Fig. 1B).
Figure 1.
Responses of RNAPII promoters to exogenously expressed TLP. (A) Amounts of overexpressing proteins in HeLa cells directed by HA-TLP and HA-TBP plasmids were determined using anti-TLP (for TLP) and anti-HA (for TBP) antibodies. The TLP antibody detects both endogenous and exogenous TLPs. Cells in lanes 2–6 were transfected with 200, 100, 50, 25 and 0 ng of HA-TLP and 0, 25, 50, 100 and 200 ng of HA-TBP, respectively. Lane 1, without transfected DNA. (B) Effects of ectopic expression of TLP and TBP on various RNAPII promoters with (a and b) or without (c and d) a TATA element. Reporter plasmids included (a) pMLP, (b) Basic, (c) pmTdT and (d) phTdT. As for effector plasmids, cells in lanes 1–5 were simultaneously transfected with 200, 100, 50, 25 and 0 ng of pTLP and 0, 25, 50, 100 and 200 ng of pTBP, respectively (solid columns). Open columns (lanes 6) show results of control experiments without effector DNA. Cells were harvested and luciferase activity was measured. Results are shown as luciferase activities relative to that in each effector-less control experiment. The data are presented as means of multiple experiments. (C) Effects of overexpressed TLP on various kinds of activator-augmented transcription. Two kinds of TATA-containing promoters (lanes 1–3 for MLP and lanes 4–7 for Basic) and two kinds of TATA-less promoters (lanes 8–10 for mTdT and lanes 11–12 for hTdT) were used as indicated. Transcriptional activators fused with the Gal4 DNA-binding domain are indicated. Open and solid bars represent transcription activity without TLP and with TLP, respectively.
We further investigated the core promoter of TATA-less mTdT and hTdT genes. In contrast to TATA-containing promoters, the mTdT promoter was considerably activated by ectopic TLP expression (Fig. 1Bc). Decreased amounts of pTLP DNA resulted in a reduced degree of stimulation. The same phenomenon was also observed for hTdT (Fig. 1Bd). The same results were obtained even when we used Renilla luciferase as an internal control or COS7 cells (data not shown). We obtained equivalent results in the case of mouse inositol 1,4,5-triphosphate (IP3) receptor (IP3R) type 2 and SV40 early promoters, which lack a canonical TATA element (data not shown).
TLP modulates the level of activated transcription
Next, we examined TLP-mediated transcriptional modulation in activator-augmented transcription using various combinations of promoter and activator plasmids. A Gal4-binding site was inserted upstream of each promoter. Activator proteins were fused with the Gal4 DNA-binding domain. In the case of the MLP and Basic promoters, TLP repressed c-Jun-, CHOP- and VP16-augmented transcription to various degrees between 8 and 75% (Fig. 1C, lanes 1–7). Among them, VP16-augmented transcription for both promoters was severely inhibited by TLP (Fig. 1C, lanes 3 and 6), whereas c-Jun-augmented transcription from MLP was only slightly affected (lane 1). On the other hand, TLP exhibited significant stimulatory effects (1.5- to 2.7-fold) on both the mTdT and hTdT promoters augmented by c-Jun, CHOP and VP16 (Fig. 1C, lanes 8–12). Consequently, as was found for the basal level of transcription in the above-described experiments, TLP was shown to modulate activator-augmented transcription negatively on TATA promoters and positively on TATA-less promoters to various extents. The same results were obtained when we used COS7 cells and HA-tagged TLP (data not shown).
Analysis of TLP function using TLP-null cells
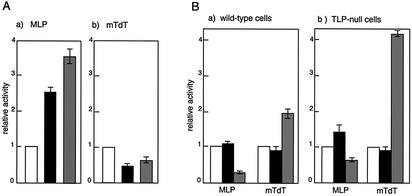
Results on TLP-mediated modulation of class II promoters were provided by an overexpression strategy. However, amounts of exogenous TLPs in cells exceeding the endogenous level (Fig. 1A, lane 2) might give rise to some artificial effects. To obtain results under more physiological conditions, we established chicken DT40 cells that lack the TLP gene and analyzed the transcription regulatory activity of TLP in those cells. The TLP-null cells propagated well (M.Shimada et al., manuscript in preparation), demonstrating that TLP is dispensable for cell growth, as was reported previously in other organisms (17,18). Compared with the degree of stimulation in normal DT40 cells, MLP promoter activity was increased by 2.5- to 3.5-fold in TLP-null cells (Fig. 2Aa), suggesting that, unlike in mammalian cells (Fig. 1Ba, lane 1), the endogenous level of TLP represses MLP in DT40 cells. In contrast, mTdT showed weakened (∼50%) promoter activity in the TLP-null cells compared with that in the wild-type cells (Fig. 2Ab). The hTdT promoter exhibited a similar phenomenon (data not shown). These results are consistent with the above-mentioned findings that exogenous TLP repressed and stimulated TATA-containing and TATA-less promoters, respectively. Based on the results shown in Figure 2A, however, it is thought that TLP is not absolutely required for the fundamental potential of the TdT promoter.
Figure 2.
Promoter strength in chicken DT40 cells that lack the TLP gene. (A) Promoter activities of TATA-carrying MLP (a) and TATA-less mTdT promoters (b) in DT40 cells were analyzed. Normal DT40 cells (open column) and two derived TLP-null cell lines, line P3 (solid columns) and line P17 (gray columns), were transfected with reporter plasmids and luciferase activities were measured. Results are shown as luciferase activities relative to those for wild-type cells. (B) Effect of TLP overexpression on promoter activity in DT40 cells. Wild-type DT40 (a) and TLP-null cells (line P3) (b) were simultaneously transfected with an effector plasmid for mTBP (solid columns) or mTLP (gray columns) and a reporter plasmid for MLP or mTdT, and luciferase activities were measured. Results are shown as luciferase activities relative to those without an effector plasmid (open columns).
We carried out TLP overexpression experiments using TLP-null DT40 cells. MLP was found to be severely repressed (by 22%) by TLP overexpression in normal DT40 cells (Fig. 2Ba). Similarly, MLP was also repressed by ectopic TLP, although the degree of repression was lower in TLP-null cells (line P3) than in wild-type cells (Fig. 2Bb). These effects of TLP overexpression on MLP activity are consistent with those in TLP-null cells (Fig. 2Aa) but are not the same as those in mammalian cells (Fig. 1Ba, lane 1). Unlike in mammalian cells, mTBP stimulated MLP only slightly in two different cell lines (Fig. 2B). On the other hand, like in mammalian cells, overexpressed TLP in the normal cells stimulated the mTdT promoter significantly (by 2.0-fold) (Fig. 2Ba), suggesting that mammalian TLP functions even in chicken cells. Notably, TLP stimulated the mTdT promoter much more (by 4.2-fold) in TLP-null cells than in the normal cells (Fig. 2Bb). This phenomenon is most likely due to no background of endogenous TLP. We obtained the same results when we used another line (P17) of TLP-null cells (data not shown). Consequently, TLP was concluded to stimulate the TATA-less TdT promoter in a transient assay condition.
Effect of a TATA element integrated in a TATA-less promoter on TLP-mediated transcriptional modulation
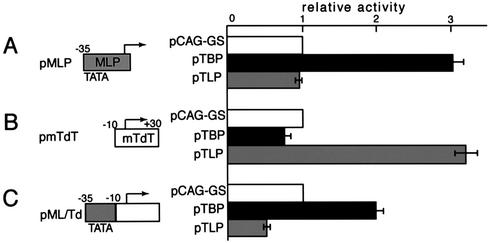
We examined the effect of a TATA element on TLP-mediated stimulation of a TATA-less promoter by using a chimeric promoter, pML/Td, consisting of the MLP TATA and mTdT core promoter (Fig. 3). Overexpressed TBP and TLP showed stimulatory effects on pMLP and pmTdT, respectively (Fig. 3A and B). However, TBP and TLP did not display an additive effect on pML/Td (Fig. 3C). pML/Td promoter activity was stimulated by TBP like pMLP but was significantly weakened by TLP. The stimulation of pML/Td by TBP is thought to be related to TBP–TATA interaction because a TATA mutation of pML/Td resulted in little stimulatory effect (data not shown). These findings imply that the TLP-mediated transcriptional modulation described above is due to the presence of a consensus TATA element.
Figure 3.
A TATA element suppresses TLP-mediated stimulation of a TATA-less promoter. Structures of reporter plasmids for MLP (pMLP) (A), mTdT promoter (pmTdT) (B) and a hybrid promoter (pML/Td) consisting of the above two elements (C) are schematically illustrated. pTLP and pTBP effector plasmids and HeLa cells were used. pCAG-GS is a control vacant plasmid. Luciferase activities are presented as ratios to those of each control experiment.
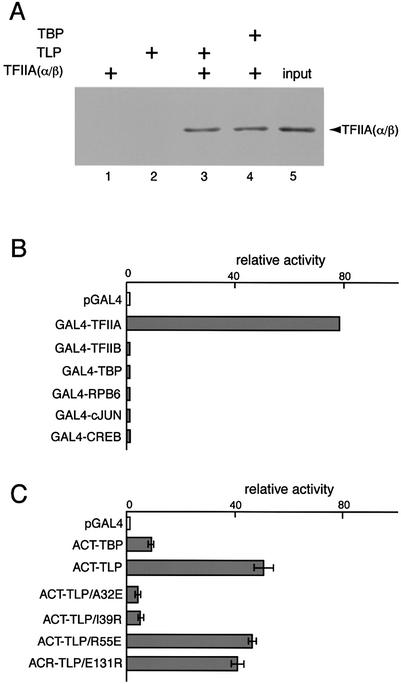
TLP is selectively associated with TFIIA
It has been shown that human TLP is associated with TFIIA in cells. The above results imply that TLP works in connection with GTFs. We performed a pull-down assay using FLAG-tagged TLP (Fig. 4A). As a control, TBP was observed to bind TFIIA (Fig. 4A, lane 4). It was clearly shown that TLP also binds to TFIIA directly (Fig. 4A, lane 3). Next, we carried out mammalian two-hybrid assays using a variety of Gal4-fusion proteins to investigate intracellular association between TLP and TFIIA. Transcriptional activators, c-Jun and CREB, did not exhibit interaction with TLP (Fig. 4B). TLP also showed no positive interaction with either RNAPII, TBP or TFIIB, whereas it showed strong interaction with the α/β subunit of TFIIA (Fig. 4B). Consequently, TLP was found to associate with TFIIA stably and selectively.
Figure 4.
Specific association of TLP with TFIIA. (A) Direct binding of TLP and TFIIA. Interaction was detected by a FLAG pull-down assay using purified TFIIA(α/β) and FLAG-tagged TLP. Eluted proteins were analyzed by western blotting with anti-TFIIA antibody (lane 3). Lanes 1 and 2 are control experiments using TFIIA and TLP alone, respectively. Lane 4, positive control experiment using TBP instead of TLP. Lane 5, 10% of the input TFIIA. (B) Mammalian cell two-hybrid assay to detect TLP binding. Interaction of TLP with the α/β subunit of TFIIA, TFIIB, TBP, RPB6 of RNAPII, c-Jun and CREB was determined using VP16 activation domain-fused TLP (ACT-TLP) and Gal4 DNA-binding domain-fused transcription factors as indicated. pGAL4 is a control vector that does not contain experimental fusion protein. (C) Interaction of TBP, TLP or mutated TLPs with TFIIA detected by the mammalian cell two-hybrid assay. A two-hybrid assay was performed by using ACT-TLP or its derivatives and GAL4-TFIIA. Residues in TLP were changed as indicated. ACT-TBP was used as a positive control.
Modified TLPs were further examined for TFIIA interaction as fused activators. All of the mutants used were confirmed to be produced equally in cells in a transient expression condition (data not shown). Notably, TLP exhibited stronger association with TFIIA than did TBP (Fig. 4C), suggesting that the stability of the TLP–TFIIA complex is greater than that of the TBP–TFIIA complex. This conclusion was confirmed by a physico-chemical determination (T.Nakadai et al., manuscript in preparation). TFIIA-related TLP mutations (ACT-TLP/A32E and ACT-TLP/I39R) carry amino acid substitutions corresponding to those amino acids of TBP that participate in TFIIA binding and subsequent transcriptional activation (31). TFIIA-related TLP mutations failed to stably associate with TFIIA, whereas two other TLP mutations (ACT-TLP/R55E and ACT-TLP/E131R) showed a similar degree of TFIIA association to that of native TLP (Fig. 4C).
Effects of TFIIA-related mutation of TLP on promoter modulation ability
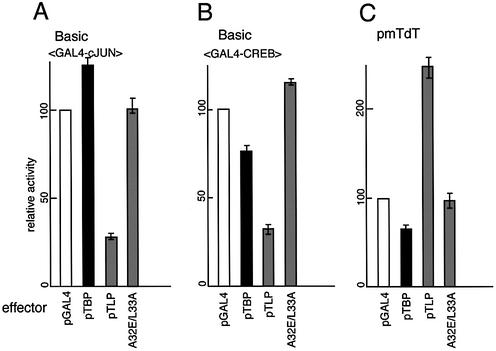
We determined how the TFIIA-binding ability of TLP affects its promoter-modulating activity using an A32E/L33A double mutant. This mutation has been confirmed not to be associated with TFIIA in cells and to be a much more severe mutation for TFIIA binding (T.Nakadai et al., manuscript in preparation). TLP inhibited activator-augmented transcription from the basic promoter (GAL4-cJUN and GAL4-CREB) as shown in Figure 1 (Fig. 5A and B). The double mutation suppressed the inhibitory effect of TLP-mediated repression of transcription from TATA-containing promoters (Fig. 5A and B). On the other hand, although TLP stimulated the TATA-less mTdT promoter, the point mutant failed to stimulate the promoter (Fig. 5C). The same phenomenon was observed in the case of other TATA-less promoters (data not shown). Consequently, TFIIA-binding ability was found to play a critical role in the promoter modulation function of TLP regardless of the type of promoter.
Figure 5.
Effects of a TFIIA-related mutation of TLP on promoter modulation ability. Repression of the activator-enhanced TATA-containing basic promoter [with GAL4-cJun (A) and GAL4-CREB (B)] and activation of the TATA-less mTdT (C) by TLP were determined using a double mutant (A32E/L33A). Results of TBP and TLP effectors are shown in the solid and gray columns, respectively. pGAL4, without effector (open columns). Plasmids for effector, reporter and activator (100 ng if necessary) were simultaneously transfected. Results are shown as relative activities.
DISCUSSION
TLP is thought to be involved in transcriptional regulation because of its TBP-like structure (14,19,20). Results of studies using TLP knock-out/knock-down animals have demonstrated a reduction in levels of transcripts of several genes (13,16–18). However, it is not clear whether TLP has an intrinsic transcription regulatory ability. In this study, we demonstrated by using a transient promoter assay as a model system that TLP modulates several RNAPII promoters. Transcriptional repression by TLP was observed in several TATA-containing promoters as previously reported for human TLP (15,21). Interestingly, TLP-mediated transcriptional stimulation was observed in the core promoter of TATA-less TdT genes. Hence, consensusness of the TATA sequence is speculated to be a key element for a response to TLP. This notion is supported by the results shown in Figure 3. We found that the SV40 early promoter and mouse IP3R type 2 promoter lacking a canonical TATA element were activated by TLP in a transient reporter assay (data not shown). We identified several TLP-responsive mouse genes (M.Shimada et al., manuscript in preparation). Interestingly, most of them are categorized as housekeeping genes and have TATA-less promoters.
Several lines of evidence suggest that the stimulatory effect on TdT promoters is thought to be mediated by TLP. Cells transfected with effector plasmids stably expressed TLP (Fig. 1A), and a point mutation of TLP impaired the stimulation function (Fig. 5). Results of assays using the mTdT promoter and TLP-null cells (Fig. 2) are essentially the same as those obtained in mammalian cells. Unlike in HeLa cells, the MLP promoter was significantly repressed by TLP in DT40 cells, both in a TLP deficiency assay condition and TLP overexpressing assay condition (Fig. 2). This discrepancy may be due to the different cells used. TLP most likely affects transcription efficiency of reporter promoters either directly or indirectly, but does not affect the efficiencies of transfection and post-transcriptional events. This contention is supported by several lines of evidence. First, TLP is physically and functionally associated with TFIIA. TLP specifically bound to TFIIA (Fig. 4), and a TLP mutant lacking TFIIA-binding ability impaired both negative and positive activities of TLP (Fig. 5). Second, joining of a TATA sequence of MLP abolished the TLP-mediated stimulation of the mTdT promoter (Fig. 3C), implying that the TATA element is a determinant of this phenomenon. Third, the results of our previous study using artificially promoter-recruited TLP (22) also suggested the importance of the intact molecular shape of TLP for its transcription stimulation activity.
Most recently, Hochheimer et al. (32) have reported the transcription activation function of Drosophila TLP (TRF2). Different from mammalian TLPs, Drosophila TLP does not bind to TFIIA but binds to DNA replication-related element-binding factor (DREF) in addition to other factors. They found that proliferating cell nuclear antigen (PCNA) is one of the targets of TLP. The Drosophila PCNA gene has two promoters: TFIID-sensitive and TFIID-insensitive promoters, the later of which has a DREF site. Notably, transcription from the TFIID-insensitive promoter was enhanced by TLP in association with DREF, whereas the TFIID-sensitive promoter was insensitive to TLP. Our notion stated here is thus consistent with their results if the TFIID-insensitive promoter of the Drosophila PCNA gene has a TATA-less-promoter function. It is noteworthy that TdT is also related to DNA synthesis like PCNA. In this study, we have demonstrated that mammalian TLP positively modulates TATA-less promoters in association with TFIIA (Fig. 5).
Although TLP is involved in transcriptional activation, it is not known how TLP works in this regulation process. Since the mutant TLP (A32E/L33A) showed no stimulation effect on the TdT promoter (Fig. 5C), it is suggested that TFIIA binding is crucial for the TLP function. TLP is thought to be recruited to a promoter via a weak interaction with the TFIIA-containing transcription machinery. TLP may function in an activator-dependent manner as has been proposed in the case of TBP (33,34). If this is the case, TFIIA is involved in hypothetical activator-directed TLP recruitment, because TFIIA is much more responsible for activator-dependent transcription than for basal transcription (26,35,36). Martinez et al. (37) suggested that TdT promoter activity depends much more on TFIIA than TATA containing that element. The promoter modulation function of TLP is thought to be determined primarily by the TATA element and TBP/TFIID occupation of it (Fig. 3), although it is still not known whether TBP (or TFIID) is required for TLP-mediated stimulation or whether TBP is substituted for TLP. We found that the mTdT promoter exhibited significant activity in TLP-null cells (Fig. 2). TLP was thus found to be dispensable for basal level transcription, and it probably acts as a modulating factor.
The TATA-containing Basic promoter was considerably repressed by TLP (Fig. 1Bb). In activator-augmented transcription, TLP constantly and strongly inhibited transcription from the MLP and Basic promoters regardless of the activator used (Fig. 1C). Moreover, TLP worked as a repressive factor for MLP in chicken DT40 cells (Fig. 2). These phenomena have been explained by the TFIIA titration-out hypothesis (15,21). However, one observation in this study cannot be explained simply by the titration-out hypothesis. As seen in lanes 2 (absence of ectopic TBP) and 3 (presence of ectopic TBP) (Fig. 1A), although the amounts of exogenous TLP in these lanes were almost the same, the basal promoter was considerably enhanced by simultaneous TBP overexpression (Fig. 1Bb). An alternative model may be needed to explain the repressive effect of TLP on TATA-containing promoters. To evaluate TLP-mediated repression of transient TATA-containing promoters, it may be necessary to determine how significantly endogenous TATA-containing promoters are influenced in a normal situation because there is much less TLP than TFIIA in cells (T.Nakadai et al., manuscript in preparation).
Acknowledgments
ACKNOWLEDGEMENT
This work was partially supported by the CREST Program of The Japan Science and Technology Corporation.
REFERENCES
- 1.Orphanides G., Lagrange,T. and Reinberg,D. (1996) The general transcription factors of RNA polymerase II. Genes Dev., 10, 2657–2683. [DOI] [PubMed] [Google Scholar]
- 2.Roeder R.G. (1996) The role of general initiation factor in transcription by RNA polymerase II. Trends Biochem. Sci., 21, 327–335. [PubMed] [Google Scholar]
- 3.Lemon B. and Tjian,R. (2000) Orchestrated response: a symphony of transcription factors for gene control. Genes Dev., 14, 2551–2569. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez N. (1993) TBP, a universal eukaryotic transcription factor? Genes Dev., 7, 1291–1308. [DOI] [PubMed] [Google Scholar]
- 5.Crowley T.E., Hoey,T., Liu,J., Jan,Y.N., Jan,L.Y. and Tjian,R. (1993) A new factor related to TATA-binding protein has highly restricted expression pattern in Drosophila. Nature, 361, 557–561. [DOI] [PubMed] [Google Scholar]
- 6.Hansen S.K., Takada,S., Jacobson,R.H., Lis,J.T. and Tjian,R. (1997) Transcription properties of a cell type-specific TATA-binding protein, TRF. Cell, 91, 71–83. [DOI] [PubMed] [Google Scholar]
- 7.Holmes M.C. and Tjian,R. (2000) Promoter-selective properties of the TBP-related factor TRF-1. Science, 288, 867–870. [DOI] [PubMed] [Google Scholar]
- 8.Takada S., Lis,J.T., Zhou,S. and Tjian,R. (2000) A TRF1:BRF complex directs Drosophila RNA polymerase III transcription. Cell, 101, 459–469. [DOI] [PubMed] [Google Scholar]
- 9.Ohbayashi T., Kishimoto,T., Makino,Y., Shimada,M., Nakadai,T., Aoki,T., Kawata,T., Niwa,S. and Tamura,T. (1999) Isolation of cDNA, chromosome mapping and expression of the human TBP-like protein. Biochem. Biophys. Res. Commun., 255, 137–142. [DOI] [PubMed] [Google Scholar]
- 10.Ohbayashi T., Makino,Y. and Tamura,T. (1999) Identification of a mouse TBP-like protein (TLP) distantly related to the Drosophila TBP-related factor. Nucleic Acids Res., 27, 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada M., Ohbayashi,T., Ishida,M., Nakadai,T., Makino,Y., Aoki,T., Kawata,T., Suzuki,T., Matsuda,Y. and Tamura,T. (1999) Analysis of the chicken TBP-like protein (tlp) gene: evidence for a striking conservation of vertebrate TLPs and for a close relationship between vertebrate tbp and tlp genes. Nucleic Acids Res., 27, 3146–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perletti L., Dantonel,J.C. and Davidson,I. (1999) The TATA-binding protein and its associated factors are differently expressed in adult mouse tissues. J. Biol. Chem., 274, 15301–15304. [DOI] [PubMed] [Google Scholar]
- 13.Kaltenbach L., Horner,M.A., Rothman,J.H. and Mango,S.E. (2000) The TBP-like factor CeTLF is required to activate RNA polymerase II transcription during C. elegans embryogenesis. Mol. Cell, 6, 705–713. [DOI] [PubMed] [Google Scholar]
- 14.Rabenstein M.D., Zhou,S., Lis,J.T. and Tjian,R. (1999) TATA box-binding protein (TBP)-related factor 2 (TRF-2), a third member of the TBP family. Proc. Natl Acad. Sci. USA, 96, 4791–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teichmann M., Wang,Z., Martinez,E., Tjernberg,A., Zhang,D., Vollmer,F., Chait,B.T. and Roeder,R.G. (1999) Human TATA-binding protein-related factor-2 (hTRF2) stably associated with hTFIIA in HeLa cells. Proc. Natl Acad. Sci. USA, 96, 13720–13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dantonel J.-C., Quintin,S., Lakatos,L., Labouesse,M. and Tora,L. (2000) TBP-like factor is required for embryonic RNA polymerase II transcription in C. elegans. Mol. Cell, 6, 715–722. [DOI] [PubMed] [Google Scholar]
- 17.Martianov I., Fimia,G.M., Dierich,A., Parvinen,M., Sassone-Corsi,P. and Davidson,I. (2001) Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP-like TLF/TRF2 gene. Mol. Cell, 7, 509–515. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D., Penttila,T.L., Morris,P.L., Teichmann,M. and Roeder,R.G. (2001) Spermiogenesis deficiency in mice lacking the trf2 gene. Science, 292, 1153–1155. [DOI] [PubMed] [Google Scholar]
- 19.Dantonel J.-C., Wurtz,J.-M., Poch,O., Moras,D. and Tora,L. (1999) The TBP-like factor: an alternative transcription factor in Metazoa? Trends Biol. Sci., 24, 335–339. [DOI] [PubMed] [Google Scholar]
- 20.Berk A.J. (2000) TBP-like factors come into focus. Cell, 103, 5–8. [DOI] [PubMed] [Google Scholar]
- 21.Moore P.A., Ozer,J., Salunek,M., Jan,G., Zerby,D., Campbell,S. and Lieberman,P.M. (1999) A human TATA binding protein-related protein with altered DNA binding specificity inhibits transcription from multiple promoters and activators. Mol. Cell. Biol., 19, 7610–7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohbayashi T., Shimada,M., Nakadai,T. and Tamura,T. (2001) TBP-like protein (TLP/TLF/TRF2) artificially recruited to a promoter stimulates basal transcription in vivo. Biochem. Biophys. Res. Commun., 285, 616–622. [DOI] [PubMed] [Google Scholar]
- 23.Niwa H., Yamamura,K. and Miyazaki,J. (1991) Efficient selection for high-expression transfection with a novel eukaryotic vector. Gene, 108, 193–200. [DOI] [PubMed] [Google Scholar]
- 24.Kato K., Makino,Y., Kishimoto,T., Yamauchi,J., Kato,S., Muramatsu,M. and Tamura,T. (1994) Multimerization of the mouse TATA-binding protein (TBP) driven by its C-terminal conserved domain. Nucleic Acids Res., 22, 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura T., Sumita,K., Hirose,S. and Mikoshiba,K. (1990) Core promoter of the mouse myelin basic protein gene governs brain-specific transcription in vitro. EMBO J., 9, 3101–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez E., Chiang,C.M., Ge,H. and Roeder,R.G. (1994) TATA-binding protein-associated factor(s) in TFIID function through the initiator to direct basal transcription from a TATA-less class II promoter. EMBO J., 13, 3115–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma D., Watanabe,H., Mermelstein,F., Admon,A., Oguri,K., Sun,X., Wada,T., Imai,T., Shiroya,T., Reinberg,D. and Handa,H. (1993) Isolation of a cDNA encoding the largest subunit of TFIIA reveals functions important for activated transcription. Genes Dev., 7, 2246–2257. [DOI] [PubMed] [Google Scholar]
- 28.Kayukawa K., Makino,Y., Yogosawa,S. and Tamura,T. (1999) A serine residue in the N-terminal acidic region of rat RPB6, one of the common subunits of RNA polymerase, is exclusively phosphorylated by casein kinase II in vitro. Gene, 234, 139–147. [DOI] [PubMed] [Google Scholar]
- 29.Li F., Takemaru,K., Goto,M., Ueda,H., Handa,H. and Hirose,S. (1997) Transcriptional activation through interaction of MBF2 with TFIIA. Genes Cells, 2, 143–153. [DOI] [PubMed] [Google Scholar]
- 30.Baba T.W., Giroir,B.P. and Humphries,E.H. (1985) Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology, 144, 139–151. [DOI] [PubMed] [Google Scholar]
- 31.Bryant G.O., Martel,L.S., Burley,S.K. and Berk,A.J. (1996) Radical mutations reveal TATA-box binding protein surfaces required for activated transcription in vivo. Genes Dev., 10, 2491–2504. [DOI] [PubMed] [Google Scholar]
- 32.Hochheimer A., Zhou,S., Zheng,S., Holmes,N.C. and Tjian,R. (2002) TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila. Nature, 420, 439–445. [DOI] [PubMed] [Google Scholar]
- 33.Kuras L. and Struhl,K. (1999) Binding of TBP to promoter in vivo is stimulated by activators and requires Pol II holoenzyme. Nature, 399, 609–613. [DOI] [PubMed] [Google Scholar]
- 34.Li X., Virbasius,A., Zhu,X. and Green,M.R. (1999) Enhancement of TBP binding by activators and general transcription factors. Nature, 399, 605–609. [DOI] [PubMed] [Google Scholar]
- 35.Pugh B.F. and Tjian,R. (1991) Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev., 5, 1935–1945. [DOI] [PubMed] [Google Scholar]
- 36.Stargell L.A. and Struhl,K. (1995) The TBP-TFIIA interaction in the response to acidic activators in vivo. Science, 269, 75–78. [DOI] [PubMed] [Google Scholar]
- 37.Martinez E., Ge,H., Tao,Y., Yuan,C., Palhan,V. and Roeder,R.G. (1998) Novel cofactors and TFIIA mediate functional core promoter selectivity by the human TAFII150-containing TFIID complex. Mol. Cell. Biol., 18, 6571–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]