Abstract
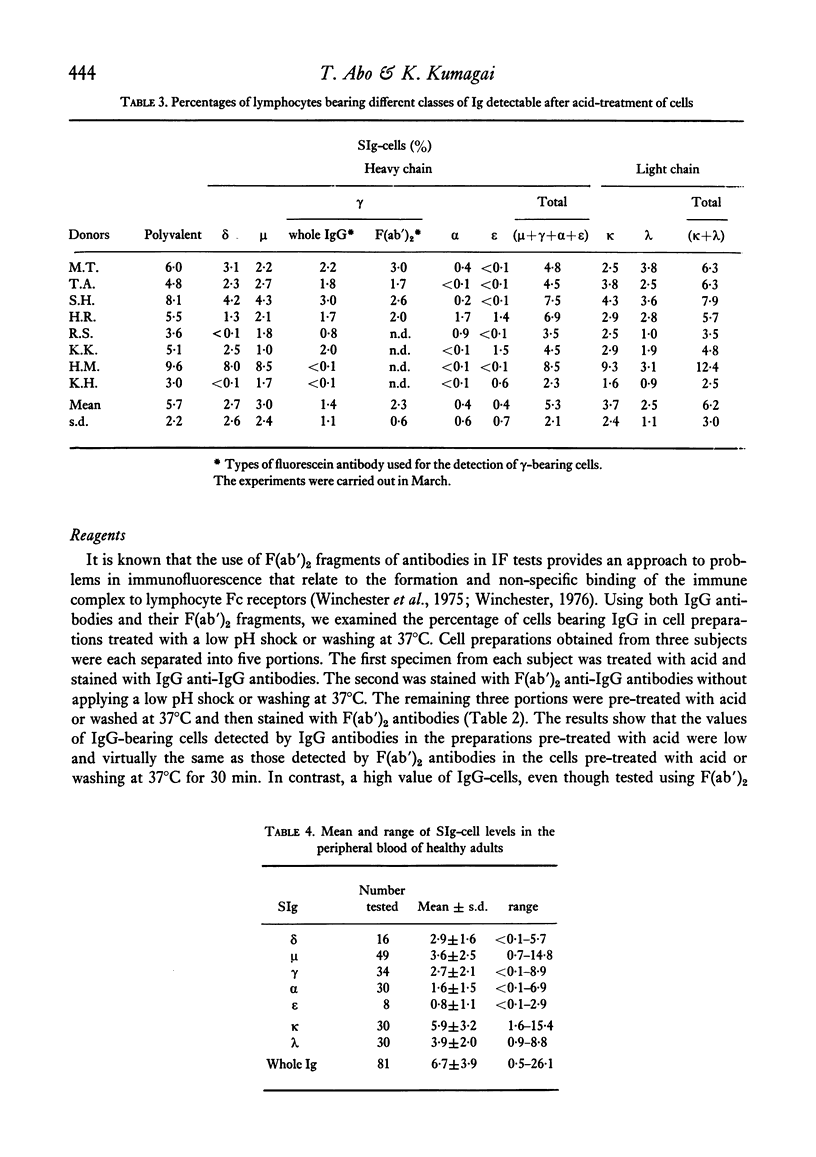
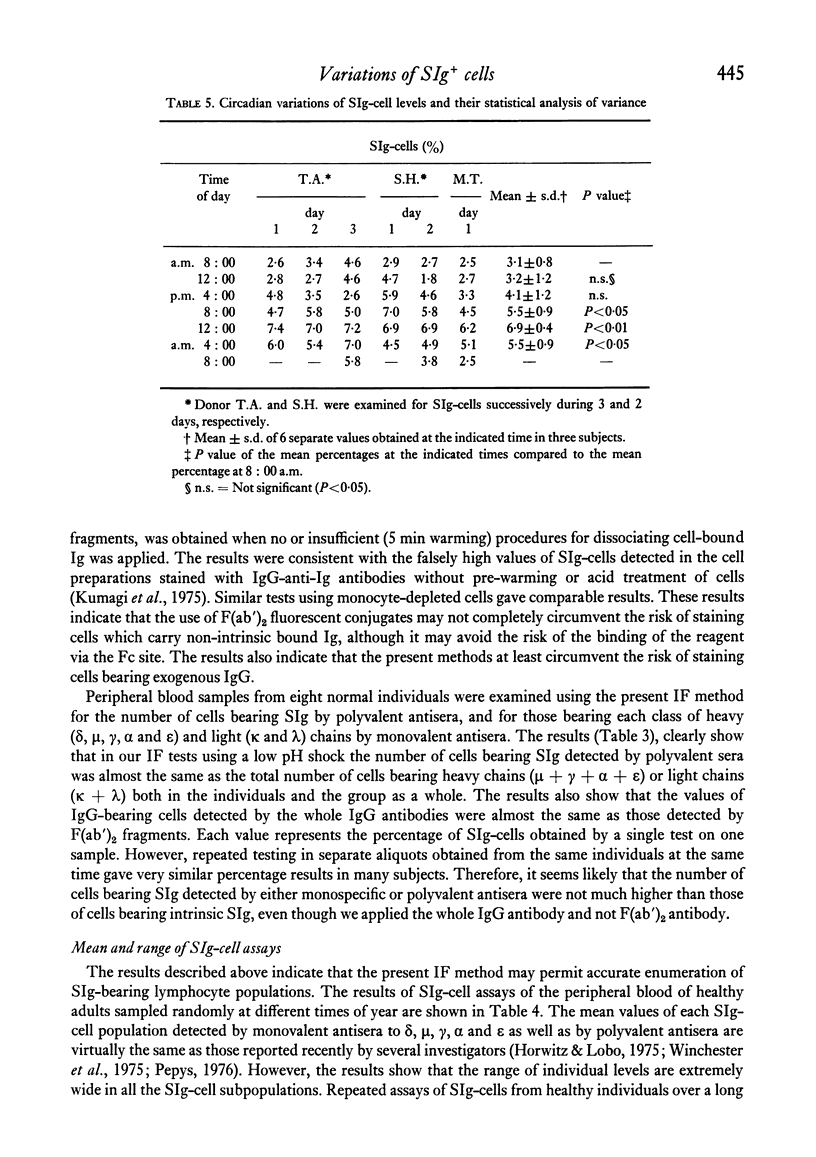
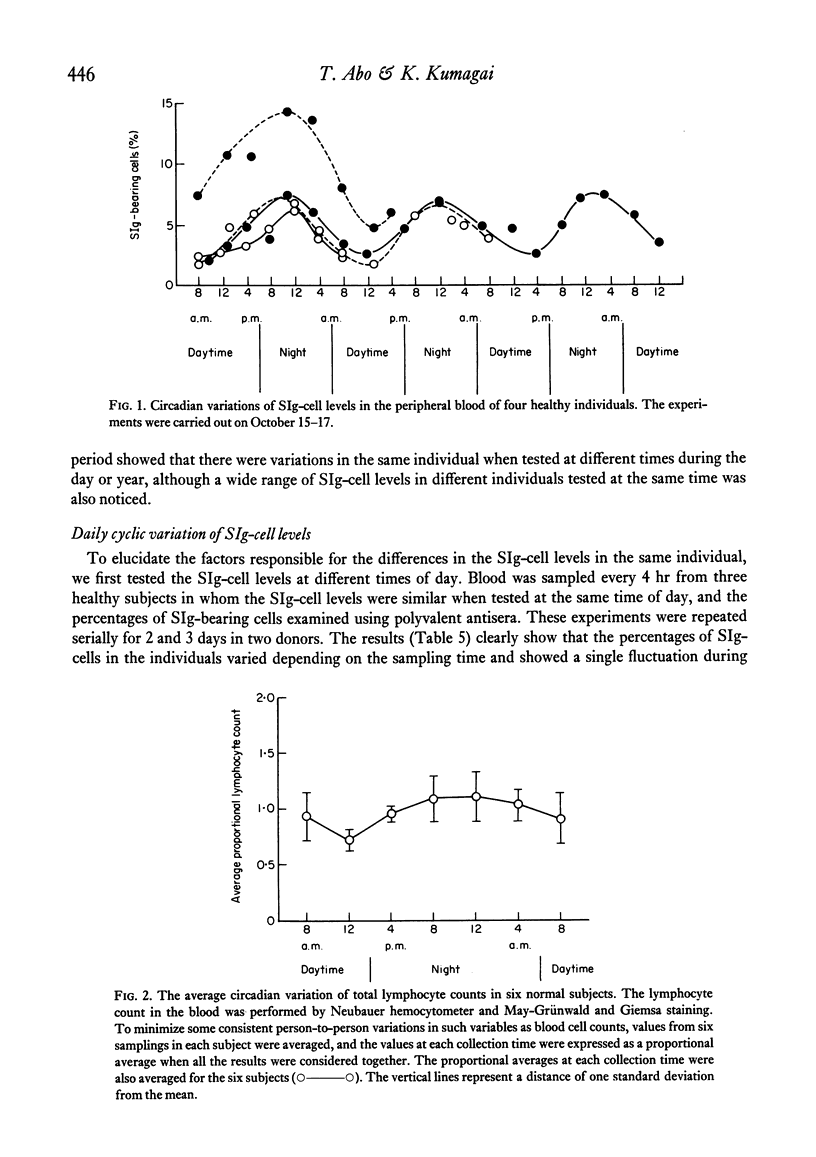
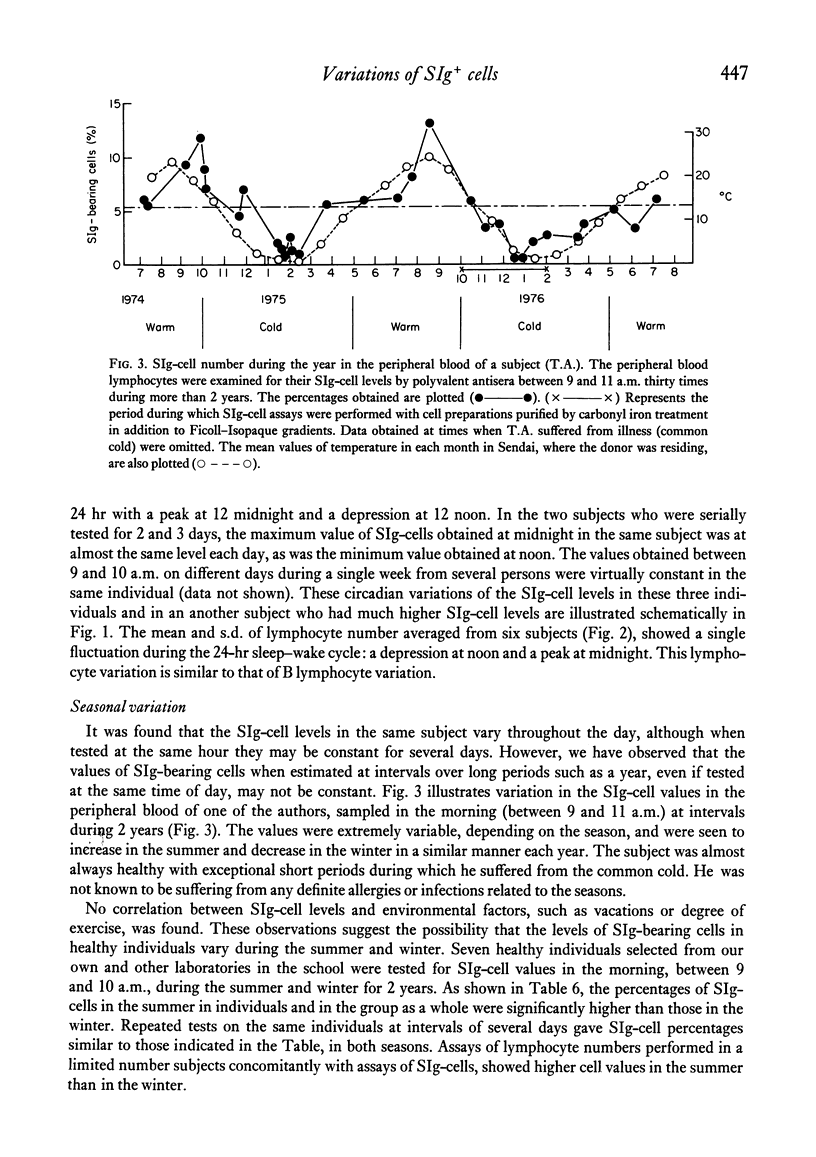
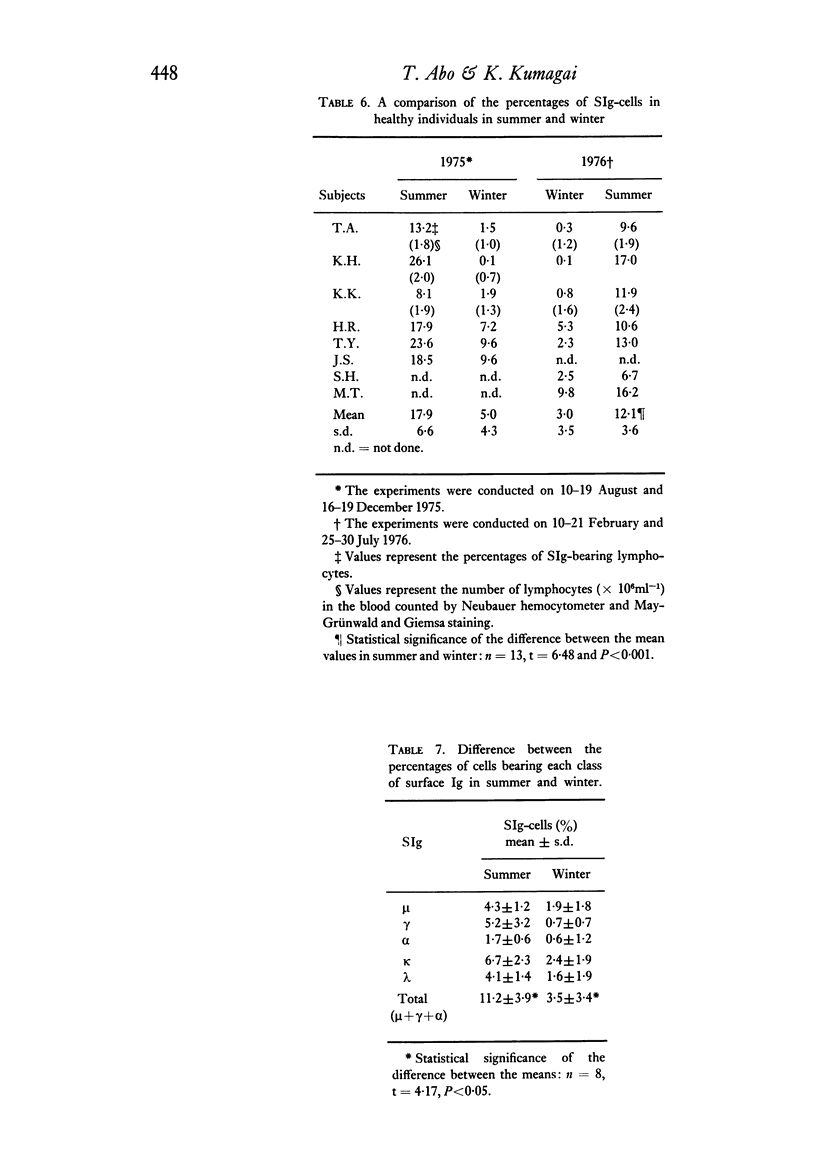
The levels of B lymphocytes bearing surface immunoglobulins in human peripheral blood were measured by immunofluorescence after pre-treatment of the cells with a low pH shock or washing at 37 degrees C. It was found that the percentages of SIg-bearing cells in an individual varied throughout the day and showed a circadian rhythm with a peak at 12 midnight and a depression at 12 noon. In addition, the levels of the B lymphocytes in the peripheral blood were shown to vary annually, being lower in the winter than in the summer.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Yamaguchi T., Shimizu F., Kumagai K. Studies of surface immunoglobulins on human B lymphocytes. II. Characterization of a population of lymphocytes lacking surface immunoglobulins but carrying Fc receptor (SIg-Fc+ cell). J Immunol. 1976 Nov;117(5 PT2):1781–1787. [PubMed] [Google Scholar]
- Carter J. B., Barr G. D., Levin A. S., Byers V. S., Ponce B., Fudenberg H. H., German D. F. Standardization of tissue culture conditions for spontaneous thymidine-2-14C incorporation by unstimulated normal human peripheral lymphocytes: circadian rhythm of DNA synthesis. J Allergy Clin Immunol. 1975 Sep;56(3):191–205. doi: 10.1016/0091-6749(75)90090-1. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Halberg F., Yunis E. J., Good R. A. Circadian rhythmic plaque-forming cell response of spleens from mice immunized with SRBC. J Immunol. 1976 Sep;117(3):962–966. [PubMed] [Google Scholar]
- Horwitz D. A., Lobo P. I. Characterizaiton of two populations of human lymphocytes bearing easily detectable surface immunoglobulin. J Clin Invest. 1975 Dec;56(6):1464–1472. doi: 10.1172/JCI108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K., Abo T., Sekizawa T., Sasaki M. Studies of surface immunoglobulins on human B lymphocytes. I. Dissociation of cell-bound immunoglobulins with acid pH or at 37 degrees C. J Immunol. 1975 Oct;115(4):982–987. [PubMed] [Google Scholar]
- MAUER A. M. DIURNAL VARIATION OF PROLIFERATIVE ACTIVITY IN THE HUMAN BONE MARROW. Blood. 1965 Jul;26:1–7. [PubMed] [Google Scholar]
- Mangi R. J., Mardiney M. R., Jr The in vitro transformation of frozen-stored lymphocytes in the mixed lymphocyte reaction and in culture with phytohemagglutinin and specific antigens. J Exp Med. 1970 Sep 1;132(3):401–416. doi: 10.1084/jem.132.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan R., Longmire R., Yelenosky R. The effect of corticosteroids on human IgG synthesis. J Immunol. 1976 Jun;116(6):1592–1595. [PubMed] [Google Scholar]
- Preud'homme J. L., Flandrin G. Identification by peroxidase staining of monocytes in surface immunofluorescence tests. J Immunol. 1974 Nov;113(5):1650–1653. [PubMed] [Google Scholar]
- Robertson W. G., Gallagher J. C., Marshall D. H., Peacock M., Nordin B. E. Seasonal variations in urinary excretion of calcium. Br Med J. 1974 Nov 23;4(5942):436–437. doi: 10.1136/bmj.4.5942.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Sekizawa T., Takahashi H., Abo T., Kumagai K. Heterogeneity of human T lymphocytes to bind sheep erythrocytes and mitogenic responses of their subpopulations. J Immunol. 1975 Dec;115(6):1509–1514. [PubMed] [Google Scholar]
- Spry C. J. Inhibition of lymphocyte recirculation by stress and corticotropin. Cell Immunol. 1972 May;4(1):86–92. doi: 10.1016/0008-8749(72)90007-x. [DOI] [PubMed] [Google Scholar]
- Stamp T. C., Round J. M. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature. 1974 Feb 22;247(5442):563–565. doi: 10.1038/247563a0. [DOI] [PubMed] [Google Scholar]
- Steel C. M., Evans J., Smith M. A. Physiological variation in circulating B cell:T cell ratio in man. Nature. 1974 Feb 8;247(5440):387–389. doi: 10.1038/247387a0. [DOI] [PubMed] [Google Scholar]
- Tavadia H. B., Fleming K. A., Hume P. D., Simpson H. W. Circadian rhythmicity of human plasma cortisol and PHA-induced lymphocyte transformation. Clin Exp Immunol. 1975 Oct;22(1):190–193. [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Fu S. M., Hoffman T., Kunkel H. G. IgG on lymphocyte surfaces; technical problems and the significance of a third cell population. J Immunol. 1975 Apr;114(4):1210–1212. [PubMed] [Google Scholar]
- Yu D. T., Clements P. J. Human lymphocyte subpopulations effect of epinephrine. Clin Exp Immunol. 1976 Sep;25(3):472–479. [PMC free article] [PubMed] [Google Scholar]


