Abstract
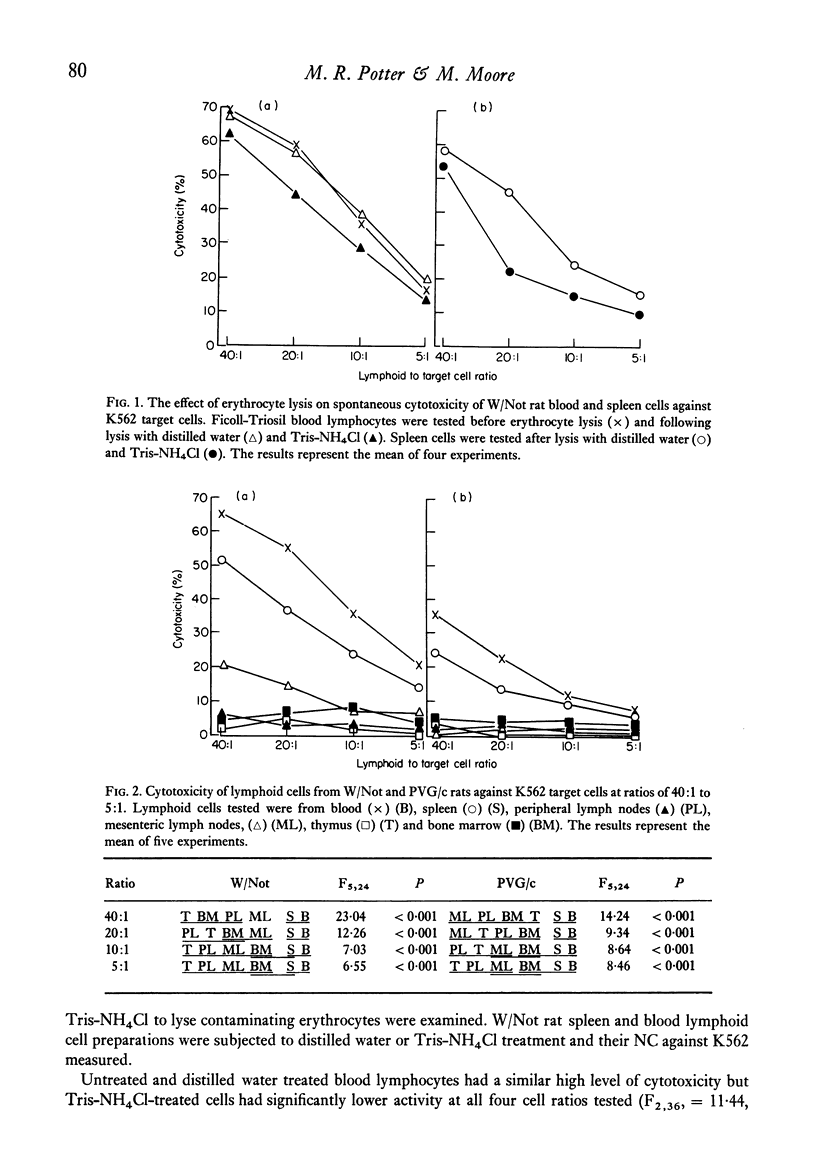
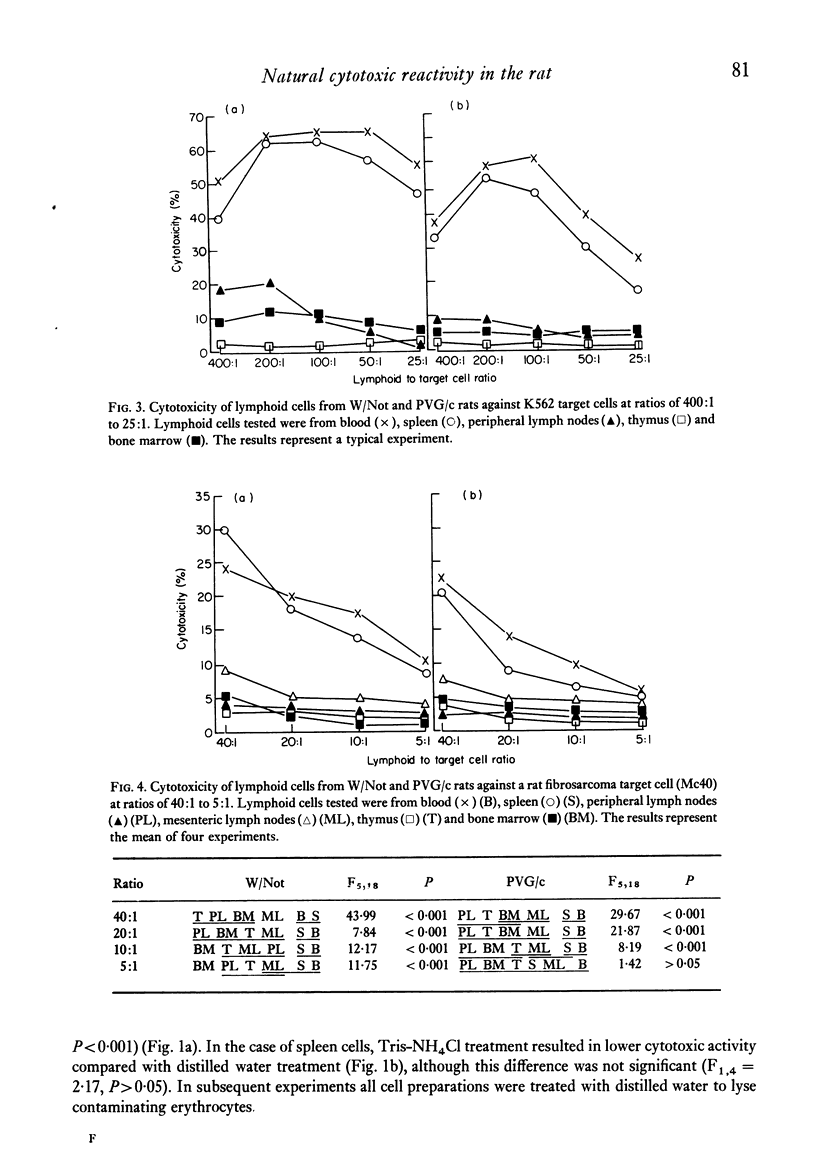
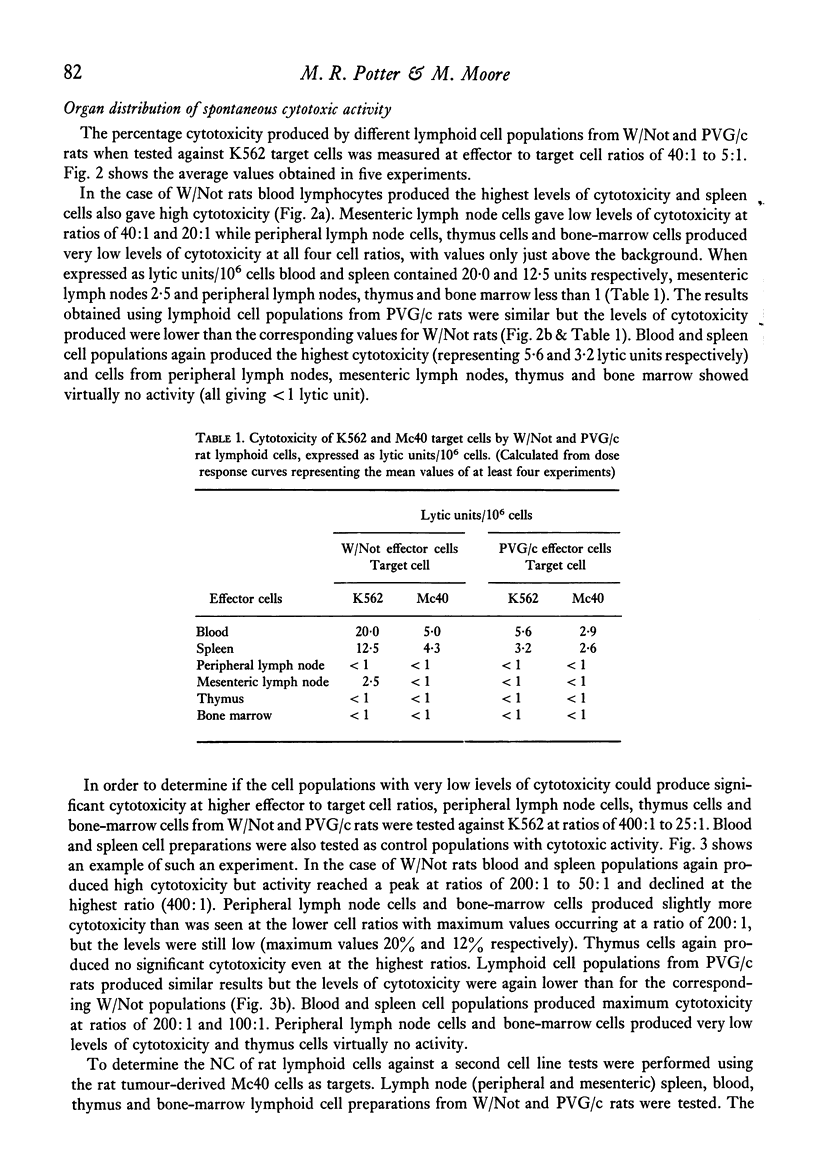
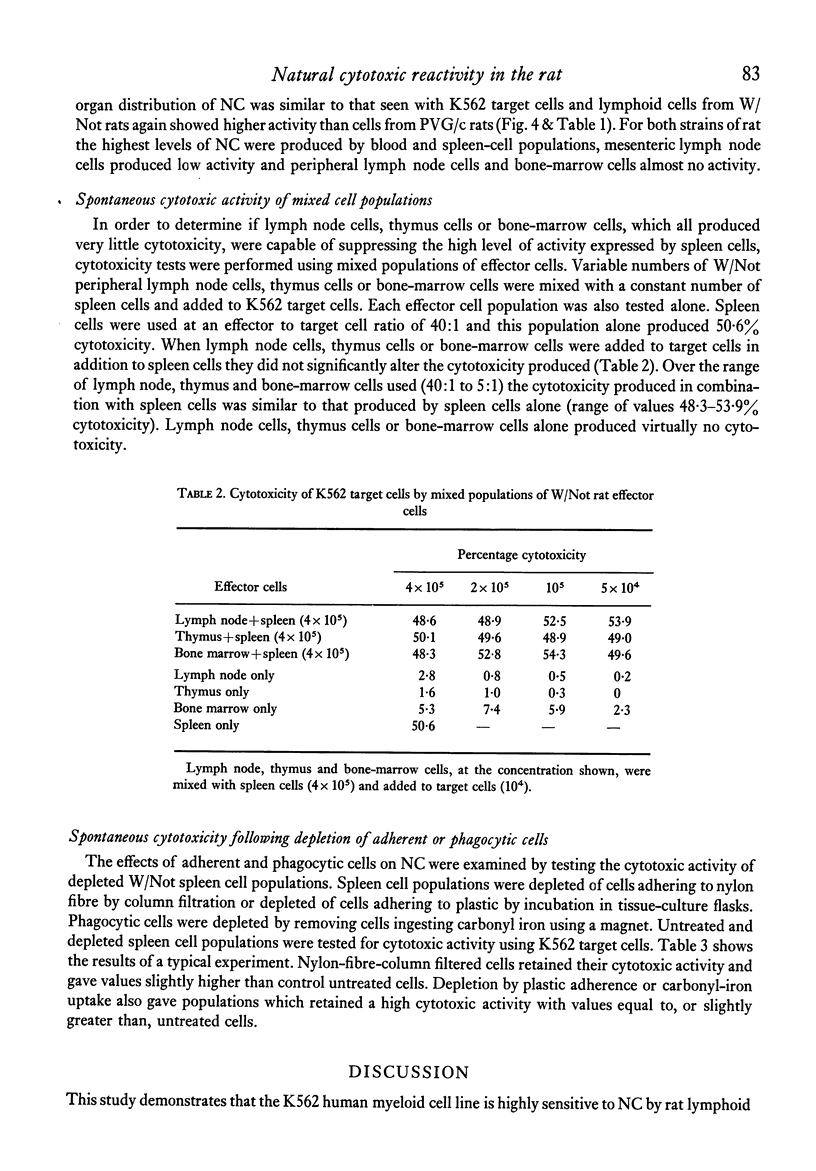
The natural (spontaneous) cytotoxicity (NC) of cell populations from different lymphoid organs of the rat were examined using a human myeloid cell line (K562) and a rat fibrosarcoma cell line (Mc40) as target cells. Rat blood and spleen lymphoid cell populations gave high cytotoxicity against K562, while lymph node cells and bone-marrow cells gave low levels of cytotoxicity and thymus cells virtually no activity. Addition of thymus or lymph node cells to spleen effector cells did not suppress the high cytotoxicity of spleen cells. A similar organ distribution of reactivity was observed against Mc40 cells, but the levels of cytotoxicity were much lower than for K562. A strain difference was monitored in the levels of natural cytotoxicity and cell populations from inbred Wistar rats consistently gave higher activity on a cell-to-cell basis than the corresponding population from PVG/c rats. Natural cytotoxicity was not removed when spleen cell populations were depleted of cells adhering to nylon-fibre columns or plastic surfaces, or depleted of cells ingesting carbonyl iron. In agreement with other studies using human and animal lymphoid cells, the natural killer cell in this system was found to be non-adherent and non-phagocytic and its distribution did not correspond to the established organ distribution of T or B lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakács T., Gergely P., Cornain S., Klein E. Characterization of human lymphocyte subpopulations for cytotoxicity against tumor-derived monolayer cultures. Int J Cancer. 1977 Apr 15;19(4):441–449. doi: 10.1002/ijc.2910190402. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Greenberg A. H., Playfair J. H. Spontaneously arising cytotoxicity to the P-815-Y mastocytoma in NZB mice. Clin Exp Immunol. 1974 Jan;16(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H., Asofsky R. Effect of antibody to theta antigen on cell-mediated immunity induced in syngeneic mice by murine sarcoma virus. J Natl Cancer Inst. 1973 Nov;51(5):1509–1512. doi: 10.1093/jnci/51.5.1509. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Jondal M., Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975 Apr 15;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kiuchi M., Takasugi M. The nonselective cytotoxic cell (N cell). J Natl Cancer Inst. 1976 Mar;56(3):575–582. doi: 10.1093/jnci/56.3.575. [DOI] [PubMed] [Google Scholar]
- Nunn M. E., Djeu J. Y., Glaser M., Lavrin D. H., Herberman R. B. Natural cytotoxic reactivity of rat lymphocytes against syngeneic Gross virus-induced lymphoma. J Natl Cancer Inst. 1976 Feb;56(2):393–399. doi: 10.1093/jnci/56.2.393. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Lindsay L. R., Nunn M. E., Herberman R. B. Natural cell-mediated cytotoxicity in rats. I. Tissue and strain distribution, and demonstration of a membrance receptor for the Fc portion of IgG. Int J Cancer. 1978 Feb 15;21(2):204–209. doi: 10.1002/ijc.2910210212. [DOI] [PubMed] [Google Scholar]
- Potter M. R., Moore M. PHA stimulation of separated human lymphocyte populations. Clin Exp Immunol. 1975 Sep;21(3):456–467. [PMC free article] [PubMed] [Google Scholar]
- Potter M. R., Moore M. The effect of adherent and phagocytic cells on human lymphocyte PHA responsiveness. Clin Exp Immunol. 1977 Jan;27(1):159–164. [PMC free article] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G. Spontaneous human lymphocyte-mediated cytotoxicity againts tumour target cells. I. The effect of malignant disease. Int J Cancer. 1976 Nov 15;18(5):593–604. doi: 10.1002/ijc.2910180508. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Jondal M. Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol. 1975 Aug;21(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E. B., McCoy J. L., Green S. S., Donnelly F. C., Siwarski D. F., Levine P. H., Herberman R. B. Destruction of human lymphoid tissue-culture cell lines by human peripheral lymphocytes in 51Cr-release cellular cytotoxicity assays. J Natl Cancer Inst. 1974 Feb;52(2):345–352. doi: 10.1093/jnci/52.2.345. [DOI] [PubMed] [Google Scholar]
- Sendo F., Aoki T., Boyse E. A., Buafo C. K. Natural occurrence of lymphocytes showing cytotoxic activity to BALB/c radiation-induced leukemia RL male 1 cells. J Natl Cancer Inst. 1975 Sep;55(3):603–609. doi: 10.1093/jnci/55.3.603. [DOI] [PubMed] [Google Scholar]
- Shellam G. R. Gross-virus-induced lymphoma in the rat. V. Natural cytotoxic cells are non-T cells. Int J Cancer. 1977 Feb 15;19(2):225–235. doi: 10.1002/ijc.2910190212. [DOI] [PubMed] [Google Scholar]
- Shellam G. R., Hogg N. Gross-virus-induced lymphoma in the rat. IV. Cytotoxic cells in normal rats. Int J Cancer. 1977 Feb 15;19(2):212–224. doi: 10.1002/ijc.2910190211. [DOI] [PubMed] [Google Scholar]
- Takasugi M., Mickey M. R., Terasaki P. I. Reactivity of lymphocytes from normal persons on cultured tumor cells. Cancer Res. 1973 Nov;33(11):2898–2902. [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- Zarling J. M., Nowinski R. C., Bach F. H. Lysis of leukemia cells by spleen cells of normal mice. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2780–2784. doi: 10.1073/pnas.72.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]


