Abstract
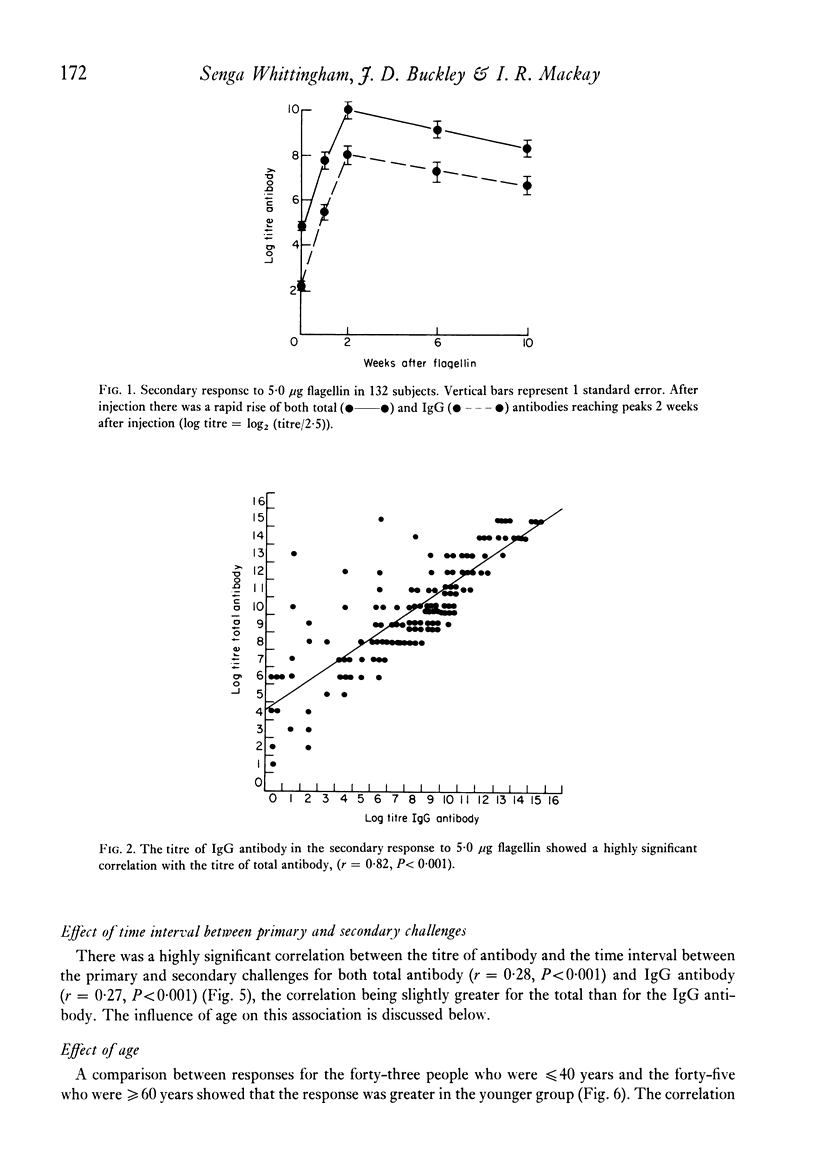
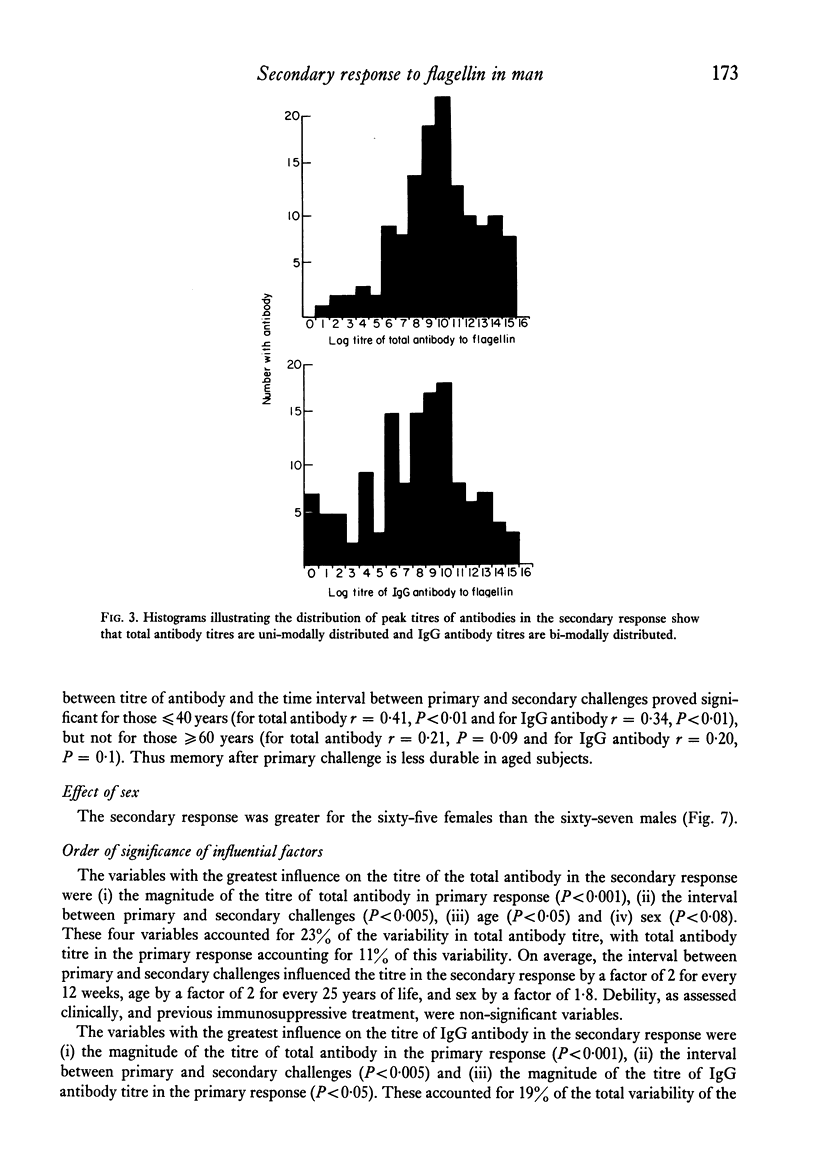
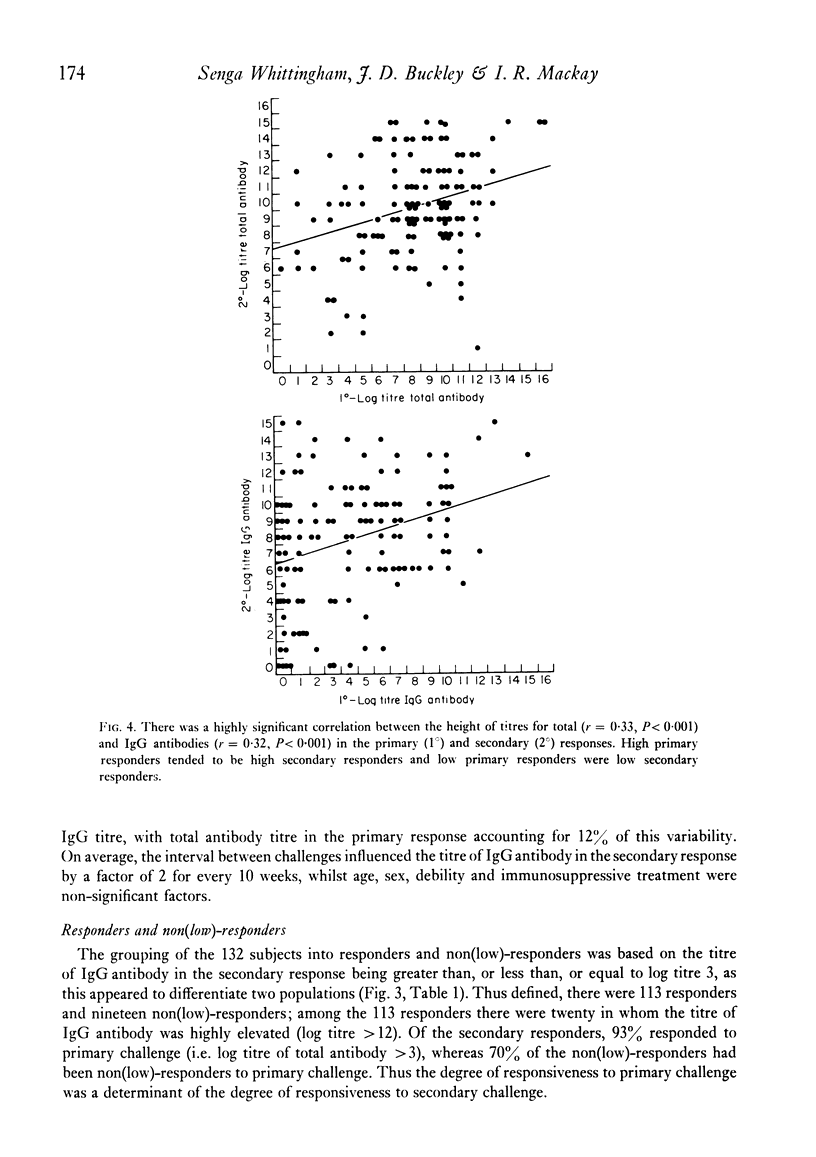
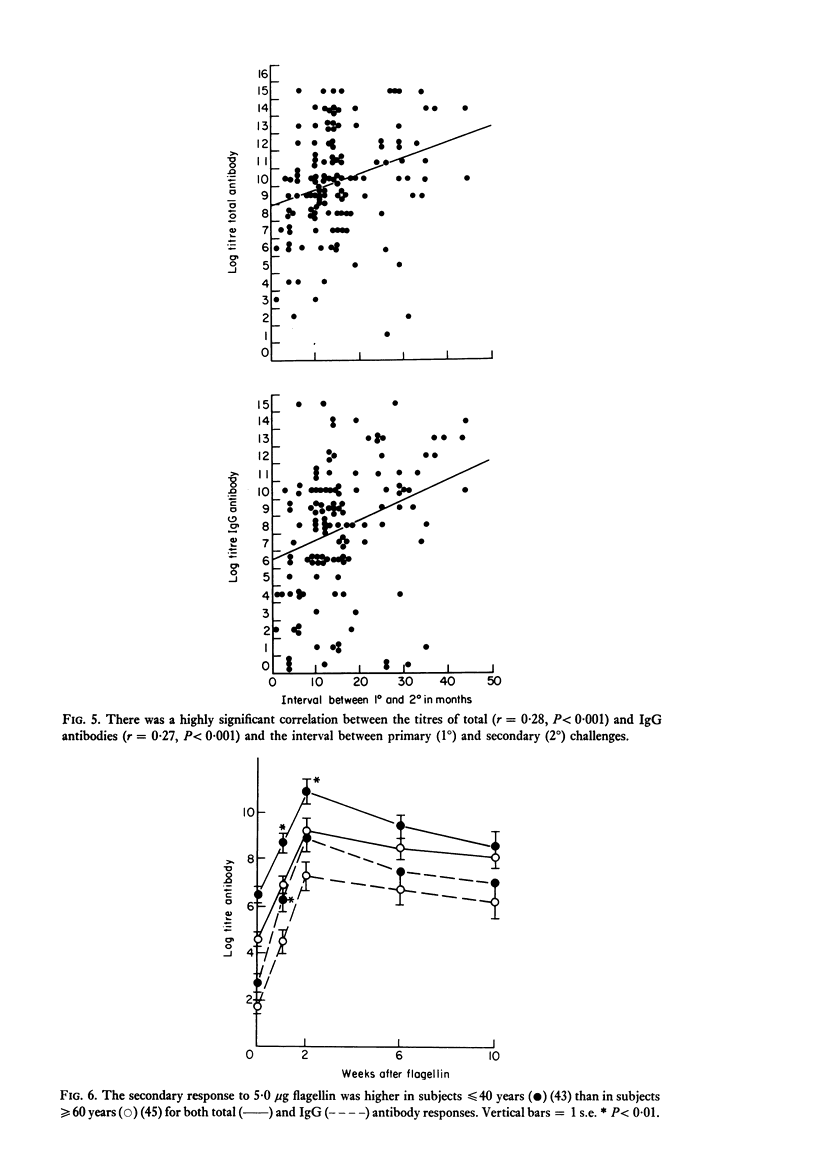
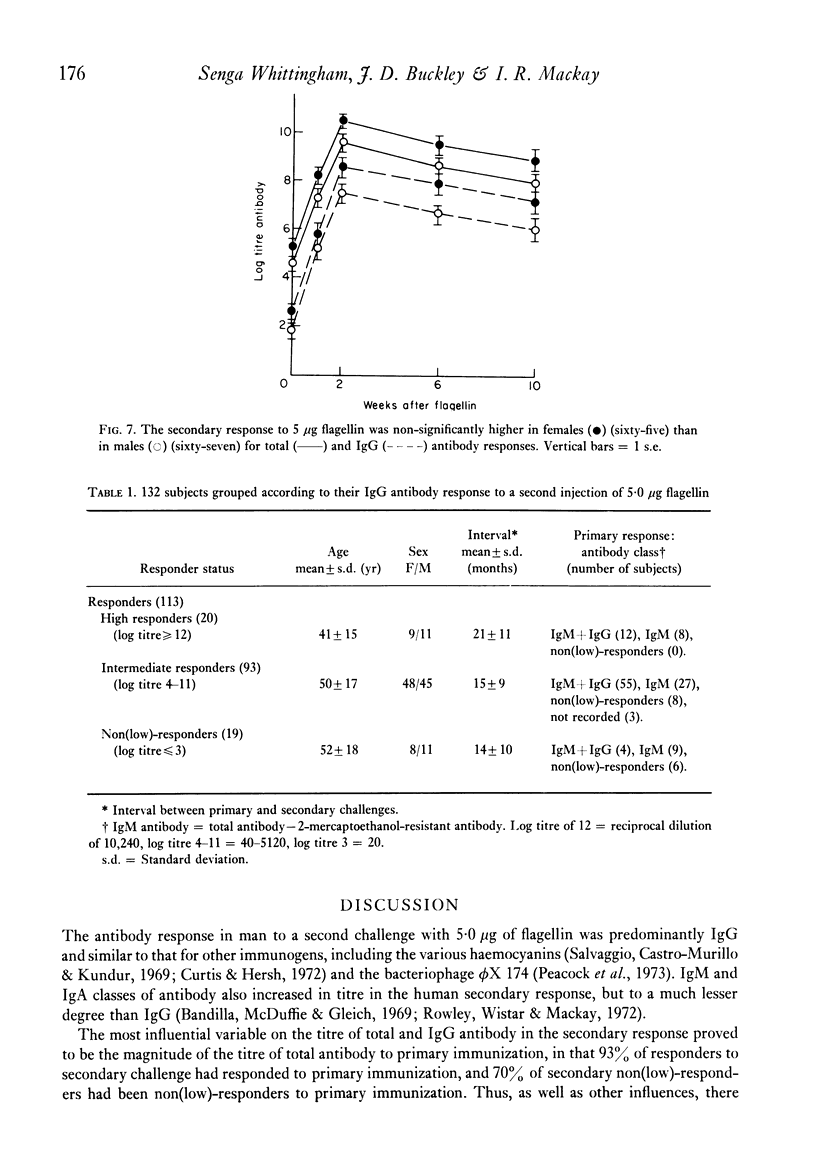
The secondary antibody response to 5.0 microgram flagellin was studied by haemagglutination in 132 healthy or convalescent subjects given a primary challenge with 5.0 microgram flagellin from 1 to 44 months previously. The peak titre, expressed as total antibody, occurred at 2 weeks and was mainly immunoglobulin (Ig)G. The magnitude of the titre of total antibody was influenced predominantly by that of total antibody in the primary response (P less than 0.001), the interval between primary and secondary responses (P less than 0.005) and the subjects' age (P less than 0.05) and sex (P less than 0.08). Together these accounted for 23% of the variability observed in the secondary response, with total antibody titre in the primary response accounting for 11% of the variability. The titre of IgG antibody was likewise influenced by these four variables, but the influence of age or sex on IgG antibody was not statistically significant. In human vaccination programmes, choice of the appropriate interval between primary and booster inoculations could increase prophylactic effectiveness and, if two inoculations were to prove as effective as three, there would be reduced work and increased public acceptance. Moreover, the demonstrable capacity for responsiveness of aged and debilitated persons should encourage the wider use of appropriate prophylactic immunization in these groups.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandilla K. K., McDuffie F. C., Gleich G. J. Immunoglobulin classes of antibodies produced in the primary and secondary responses in man. Clin Exp Immunol. 1969 Dec;5(6):627–641. [PMC free article] [PubMed] [Google Scholar]
- Curtis J. E., Hersh E. M., Harris J. E., McBride C., Freireich E. J. The human primary immune response to keyhole limpet haemocyanin: interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. Clin Exp Immunol. 1970 Apr;6(4):473–491. [PMC free article] [PubMed] [Google Scholar]
- Curtis J. E., Hersh E. M. The human secondary immune response to Keyhole limpet haemocyanin. Clin Exp Immunol. 1972 Jan;10(1):171–177. [PMC free article] [PubMed] [Google Scholar]
- Edsall G., Belsey M. A., LeBlanc D. R., Levine L. Host factors in the response to immunization. Prog Drug Res. 1975;19:263–273. doi: 10.1007/978-3-0348-7090-0_30. [DOI] [PubMed] [Google Scholar]
- Lee A. K., Mackay I. R., Rowley M. Measurement of antibody-producing capacity in man. 3. The response to flagellin from Salmonella adelaide in chronic diseases. Int Arch Allergy Appl Immunol. 1971;41(2):358–369. doi: 10.1159/000230531. [DOI] [PubMed] [Google Scholar]
- Mathews J. D., Mackay I. R., Whittingham S., Malcolm L. A. Protein supplementation and enhanced antibody-producing capacity in New Guinean school-children. Lancet. 1972 Sep 30;2(7779):675–677. doi: 10.1016/s0140-6736(72)92086-7. [DOI] [PubMed] [Google Scholar]
- Morris P. J., Vaughan H., Tait B. D., Mackay I. R. Histocompatibility antigens (HLA): associations with immunopathic diseases and with responses to microbial antigens. Aust N Z J Med. 1977 Dec;7(6):616–624. doi: 10.1111/j.1445-5994.1977.tb02318.x. [DOI] [PubMed] [Google Scholar]
- Newell K. W., Leblanc D. R., Edsall G., Levine L., Christensen H., Montouri M. H., Ramirez N. The serological assessment of a tetanus toxoid field trial. Bull World Health Organ. 1971;45(6):773–785. [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Benacerraf B. Functional specificity of thymus- dependent lymphocytes. Science. 1977 Mar 25;195(4284):1293–1300. doi: 10.1126/science.320663. [DOI] [PubMed] [Google Scholar]
- Peacock D. B., Jones J. V., Gough M. The immune response to thetaX 174 in man. I. Primary and secondary antibody production in normal adults. Clin Exp Immunol. 1973 Apr;13(4):497–513. [PMC free article] [PubMed] [Google Scholar]
- Pereira M. S. The role of epidemiological surveillance in the immunoprophylaxis of influenza. Postgrad Med J. 1976 Jun;52(608):323–326. doi: 10.1136/pgmj.52.608.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Whittingham S., Youngchaiyud U., Mackay I. R. Ageing, immune response, and mortality. Lancet. 1974 Aug 17;2(7877):368–370. doi: 10.1016/s0140-6736(74)91755-3. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Mackay I. R. Measurement of antibody-producing capacity in man. I. The normal response to flagellin from Salmonella adelaide. Clin Exp Immunol. 1969 Oct;5(4):407–418. [PMC free article] [PubMed] [Google Scholar]
- Rowley M. J., Wistar R., Mackay I. R. Measurement of antibody-producing capacity in man. V. Immunoglobulin classes of antibodies to flagellin. Immunology. 1972 Mar;22(3):475–484. [PMC free article] [PubMed] [Google Scholar]
- Salvaggio J., Castro-Murillo E., Kundur V. Immunologic response of atopic and normal individuals to keyhole limpet hemocyanin. J Allergy. 1969 Dec;44(6):344–354. doi: 10.1016/0021-8707(69)90026-4. [DOI] [PubMed] [Google Scholar]
- Wells J. V., Fudenberg H. H., MacKay I. R. RElation of the human antibody response to flagellin to GM genotype. J Immunol. 1971 Dec;107(6):1505–1511. [PubMed] [Google Scholar]
- Wistar R., Jr Serum antibody to Salmonella flagellar antigens. I. Methods of antibody assay. Aust J Exp Biol Med Sci. 1968 Dec;46(6):769–777. doi: 10.1038/icb.1968.183. [DOI] [PubMed] [Google Scholar]
- de Gast G. C., The T. H., Snijder J. A. The human immune response to alpha-haemocyanin of Helix pomatia. Acta Med Scand. 1973 Oct;194(4):303–309. doi: 10.1111/j.0954-6820.1973.tb19450.x. [DOI] [PubMed] [Google Scholar]



