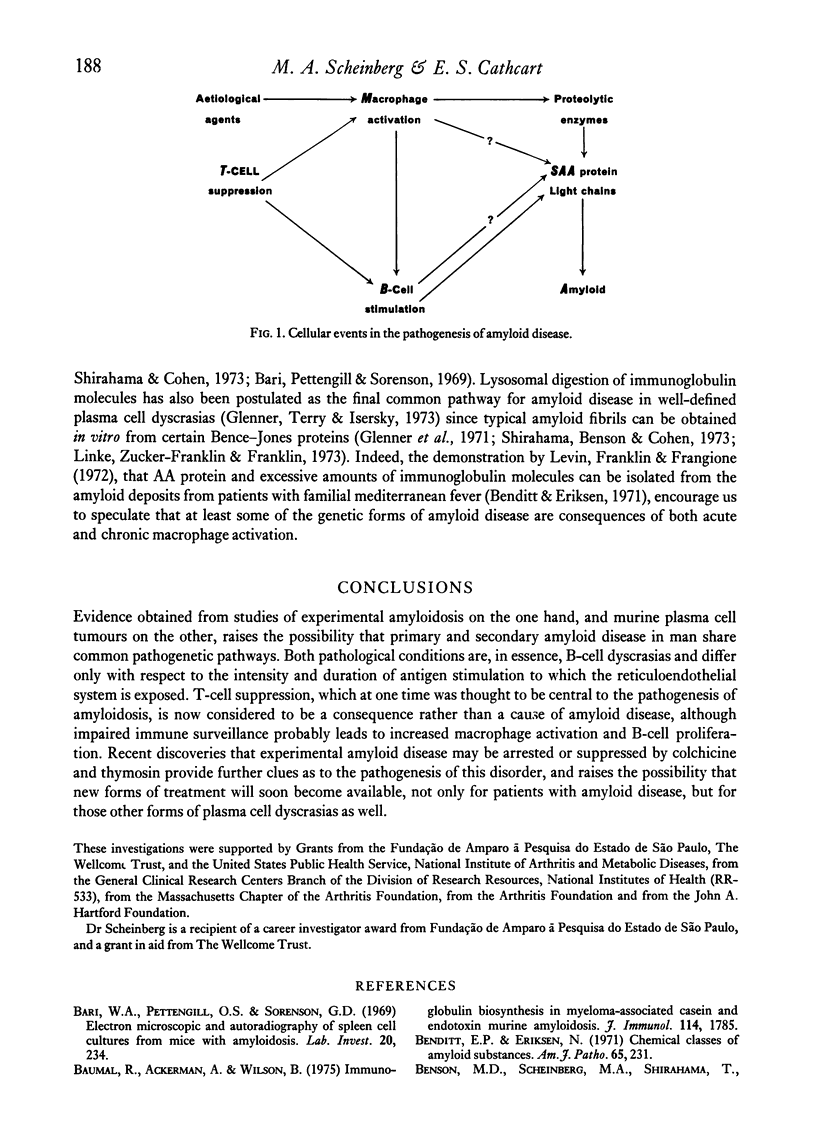
Abstract
Despite the marked progress obtained in the structural and amino acid sequencing data of amyloid proteins our understanding of the cellular mechanisms causing the deposition of amyloid fibrils is still poor. Some of the questions about the cellular events leading to the synthesis of amyloid fibrils can be approached by evaluating the immune reactivity of animals that develop amyloid after repeated daily casein injections. Recent studies carried out in a mouse model indicate that macrophage activation associated with T-cell suppression and followed by B-cell proliferation appear to be responsible for the immunopathological abnormalities in both primary and secondary amyloid disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bari W. A., Pettengill O. S., Sorenson G. D. Electron microscopy and electron microscopic autoradiography of splenic cell cultures from mice with amyloidosis. Lab Invest. 1969 Mar;20(3):234–242. [PubMed] [Google Scholar]
- Baumal R., Ackermann A., Wilson B. Immunoglobulin biosynthesis in myeloma-associated and casein- and endotoxin-induced murine amyloidosis. J Immunol. 1975 Jun;114(6):1785–1791. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical classes of amyloid substance. Am J Pathol. 1971 Oct;65(1):231–252. [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Scheinberg M. A., Shirahama T., Cathcart E. S., Skinner M. Kinetics of serum amyloid protein A in casein-induced murine amyloidosis. J Clin Invest. 1977 Mar;59(3):412–417. doi: 10.1172/JCI108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton S. Experimental amyloidosis: the inducer is a polyclonal B-cell activator to which susceptibility is under genetic control. J Exp Med. 1975 Dec 1;142(6):1564–1569. doi: 10.1084/jem.142.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- COHEN A. S., CALKINS E. Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature. 1959 Apr 25;183(4669):1202–1203. doi: 10.1038/1831202a0. [DOI] [PubMed] [Google Scholar]
- Calderon J., Williams R. T., Unanue E. R. An inhibitor of cell proliferation released by cultures of macrophages. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4273–4277. doi: 10.1073/pnas.71.11.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart E. S., Mullarkey M., Cohen A. S. Cellular immunity in casein-induced amyloidosis. Immunology. 1971 Jun;20(6):1001–1008. [PMC free article] [PubMed] [Google Scholar]
- Cohen A. S. Amyloidosis. N Engl J Med. 1967 Sep 7;277(10):522–contd. doi: 10.1056/NEJM196709072771006. [DOI] [PubMed] [Google Scholar]
- Druet R. L., Janigan D. T. Experimental amyloidosis. Rates of induction, lymphocyte depletion and thymic atrophy. Am J Pathol. 1966 Nov;49(5):911–929. [PMC free article] [PubMed] [Google Scholar]
- Ebbesen P. Mutually exclusive occurrence of amyloidosis and thymic leukaemia in casein treated AKR mice. Br J Cancer. 1974 Jan;29(1):76–79. doi: 10.1038/bjc.1974.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen N., Ericsson L. H., Pearsall N., Lagunoff D., Benditt E. P. Mouse amyloid protein AA: Homology with nonimmunoglobulin protein of human and monkey amyloid substance. Proc Natl Acad Sci U S A. 1976 Mar;73(3):964–967. doi: 10.1073/pnas.73.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin E. C., Zucker-Franklin D. Current concepts of amyloid. Adv Immunol. 1972;15:249–304. doi: 10.1016/s0065-2776(08)60687-2. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Ein D., Eanes E. D., Bladen H. A., Terry W., Page D. L. Creation of "amyloid" fibrils from Bence Jones proteins in vitro. Science. 1971 Nov 12;174(4010):712–714. doi: 10.1126/science.174.4010.712. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W. D., Isersky C. Amyloidosis: its nature and pathogenesis. Semin Hematol. 1973 Jan;10(1):65–86. [PubMed] [Google Scholar]
- Hardt F., Claësson M. H. Studies on casein-induced amyloidosis in mice with congenital aplasia of the thymus. Acta Pathol Microbiol Scand A. 1972;80(4):471–476. doi: 10.1111/j.1699-0463.1972.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- KELLUM M. J., SUTHERLAND D. E., ECKERT E., PETERSON R. D., GOOD R. A. WASTING DISEASE, COOMBS-POSITIVITY, AND AMYLOIDOSIS IN RABBITS SUBJECTED TO CENTRAL LYMPHOID TISSUE EXTIRPATION AND IRRADIATION. Int Arch Allergy Appl Immunol. 1965;27:6–26. doi: 10.1159/000229594. [DOI] [PubMed] [Google Scholar]
- Kedar I., Ravid M., Sohar E., Gafni J. Colchicine inhibition of casein-induced amyloidosis in mice. Isr J Med Sci. 1974 Jul;10(7):787–789. [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limas C., Wright J. R., Matsuzaki M., Calkins E. Amyloidosis and multiple myeloma. A reevaluation using a control population. Am J Med. 1973 Feb;54(2):166–173. doi: 10.1016/0002-9343(73)90220-9. [DOI] [PubMed] [Google Scholar]
- Mandel M. A., DeCosse J. J. The effects of heterologous antithymocyte sera in mice. V. Enhancement of plasma cell tumor induction. J Immunol. 1972 Aug;109(2):360–365. [PubMed] [Google Scholar]
- Namba Y., Hanaoka M. Immunocytology of cultured IgM-forming cells of mouse. I. Requirement of phagocytic cell factor for the growth of IgM-forming tumor cells in tissue culture. J Immunol. 1972 Dec;109(6):1193–1200. [PubMed] [Google Scholar]
- Parker J. W., Metcalf D. Production of colony-stimulating factor in mitogen-stimulated lymphocyte cultures. J Immunol. 1974 Feb;112(2):502–510. [PubMed] [Google Scholar]
- Potter M. The developmental history of the neoplastic plasma cell in mice: a brief review of recent developments. Semin Hematol. 1973 Jan;10(1):19–32. [PubMed] [Google Scholar]
- Scheinberg M. A., Bennett M., Cathcart E. S. Casein-induced experimental amyloidosis. V. The response of lymphoid organs to T and B mitogens. Lab Invest. 1975 Jul;33(1):96–101. [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S. Casein-induced experimental amyloidosis. III. Response to mitogens, allogeneic cells, and graft-versus-host reactions in the murine model. Immunology. 1974 Nov;27(5):953–963. [PMC free article] [PubMed] [Google Scholar]
- Scheinberg M. A., Goldstein A. L., Cathcart E. S. Thymosin restores T cell function and reduces the incidence of amyloid disease in casein-treated mice. J Immunol. 1976 Jan;116(1):156–158. [PubMed] [Google Scholar]
- Shinohara N., Okumura K., Kern M. Differentiation of lymphoid cells: the non-mitogenic induction of immunoglobulin production by thymus cell extract and thymus cell culture filtrate. Immunology. 1976 Sep;31(3):433–441. [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Benson M. D., Cohen A. S., Tanaka A. Fibrillar assemblage of variable segments of immunoglobulin light chains: an electron microscopic study. J Immunol. 1973 Jan;110(1):21–30. [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. An analysis of the close relationship of lysosomes to early deposits of amyloid. Ultrastructural evidence in experimental mouse amyloidosis. Am J Pathol. 1973 Oct;73(1):97–114. [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. Blockage of amyloid induction by colchicine in an animal model. J Exp Med. 1974 Oct 1;140(4):1102–1107. doi: 10.1084/jem.140.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S., Scheinberg M. A., Yonkosky D., Cathcart E. S. Monocyte involvement in PHA induced cytotoxicity of chicken erythrocytes in man. Cell Immunol. 1976 Mar 1;22(1):93–97. doi: 10.1016/0008-8749(76)90010-1. [DOI] [PubMed] [Google Scholar]
- Skinner M., Cathcart E. S., Cohen A. S., Benson M. D. Isolation and identification by sequence analysis of experimentally induced guinea pig amyloid fibrils. J Exp Med. 1974 Sep 1;140(3):871–876. doi: 10.1084/jem.140.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]


