Abstract
A novel octahedral complex CoII(HAPP)(TFA)2 [hexaazaphenantholine-cyclophane (HAPP), trifluoroacetate (TFA)] is a DNA bulge-specific probe with single-strand DNA cleavage activity in the presence of H2O2. This complex exhibits low affinity towards double-stranded DNA and low reactivity toward single-stranded DNA. Metal–HAPP complexes with different coordination number and ring size were synthesized and their selectivity and reactivity for DNA bulges were compared. The DNA sequence at the bulge site influences the intensity of cleavage at the bulge and the flanking sites after piperidine treatment. Cleavage specificity of CoII(HAPP)(TFA)2 was characterized extensively using scavenger reagents to quench the cleavage reaction and high-resolution polyacrylamide gel electrophoresis. In addition, 3′-phosphoglycolate cleavage products were trapped and analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. These data were used to deduce that the DNA cleavage pathway for CoIIHAPP2+ in the presence of H2O2 involves 4′-H abstraction of the deoxyribose moiety.
INTRODUCTION
Much insight into genetic events and processes is gained by studying interactions between nucleic acids and molecules that bind to or modify nucleic acids. The biological function of many drugs and biopolymers requires highly specific nucleic acid binding which is facilitated by recognition of unique nucleobase sequences or structural elements. Structural elements recognized by nucleic acid binding molecules include unobstructed single-stranded sites, bulges, hairpin loops, internal loops and double-stranded helical junctions. DNA sequences carry essential genetic information, so DNA sequence changes can be lethal if they alter gene expression or produce a non-functional variant of an essential protein. Bulges are a common structural element in RNA, but are less common in DNA, and were believed to occur primarily as transient intermediates in frameshift mutagenesis (1–3). The unpaired nucleobases of bulges are capable of forming complexes with nucleic acid-binding proteins and are the targets of small DNA-binding molecules (4–6). It has been difficult to obtain detailed structural information about DNA bulges, because they are relatively unstable (7,8). Nevertheless, a few DNA bulges have been successfully analyzed by X-ray crystallography (9,10) or NMR (11,12).
Highly specific chemical nucleases have been very useful probes of the structure of nucleic acid bulges. Many chemical nucleases nick specific sequences or nucleotides in DNA or RNA (13,14). In addition, a small number of transition metal complexes have been demonstrated that recognize and cleave the single-stranded nucleotides in DNA and RNA bulges. Recently, a novel octahedral complex, CoII(HAPP)(TFA)2 [hexaazaphenantholine-cyclophane (HAPP), trifluoroacetate (TFA)], was identified that specifically cleaves DNA bulges (15). This complex shows low affinity towards and does not cleave double-stranded or single-stranded DNA.
This work examines the mechanism by which CoIIHAPP2+ cleaves DNA bulges and compares the reactivity and specificity of several metal complexes with different geometries. The DNA sequence-dependence of cleavage was also examined. The DNA cleavage mechanism was investigated using high-resolution polyacrylamide gel electrophoresis and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The results suggest that CoIIHAPP2+ causes ribose–sugar oxidation at the 4′-H position in the presence of hydrogen peroxide.
MATERIALS AND METHODS
All reagents and ACS grade solvents were purchased from commercial sources and were used without further purification unless otherwise noted. CoIIHAPP2+, CuIIHAPP2+ and NiIIHAPP2+ complexes were prepared according to a published procedure (16). UV-Vis spectra were obtained in H2O and/or in 10 mM phosphate buffer on a Hewlett-Packard 8453 diode array spectrometer. λ-Phage ΦX-174 supercoiled plasmid DNA was purchased from Life Technologies (Gibco BRL). No purification was needed prior to use. Synthetic oligonucleotides were purchased from Biobasic Inc. (Canada) and Life Technologies (Gibco BRL).
Cleavage of supercoiled DNA
DNA cleavage was assayed in a volume of 10 µl containing ΦX-174 supercoiled plasmid DNA (250 ng, ∼60 µM nucleotide) mannitol (0, 25 and 50 mM) and 10 mM sodium phosphate (pH 7). CoHAPP2+ (0.6 µM) and H2O2 (0.01%) were added sequentially and the reaction was incubated at 25°C for 5–10 min. The reaction was quenched with gel-loading solution containing bromophenol blue (0.25%), xylene cyanol FF (0.25%) and glycerol (50%). Form I (supercoiled) and Form II (circular) DNA were separated by agarose gel electrophoresis (1% agarose, 1× TAE), stained with ethidium bromide (0.1 µg/ml), destained with deionized H2O for 30 min, and photographed with Kodak Digital Science EDAS.
DNA preparation
Synthetic oligonucleotides were purified on a 20% denaturing polyacrylamide gel (7 M urea). The DNA bands were visualized by placing the gel on the top of a TLC F254 plate (20 × 20 cm, Merck) and exposing it to UV light (λmax 254 nm). The full-length oligonucleotide was excised from the gel, extracted and precipitated with ethanol. The DNA concentration was estimated by absorption spectroscopy at λmax 260 nm using the extinction coefficient or by a calculation based on average nucleic acid properties (1 OD ≈ 33 µg/ml single-stranded DNA; the average molecular weight of one nucleotide = 330 Da). Oligomers were 5′-32P-end-labeled using T4 polynucleotide kinase (New England Biolabs) and deoxyadenosine-5′-[γ-32P]triphosphate (Du Pont). Excess [γ-32P]ATP was removed by filtration with Centricon-10 (Amicon) using an ultracentrifuge (6000 r.p.m., Beckmann GS-15R equipped with rotor F0850) at 4°C for 80 min, followed by an additional centrifugation with Milli-Q water (1 ml) for 60 min. The samples were diluted to the desired concentration with deionized water for cleavage reactions with metal complexes.
Double-stranded oligomers were constructed by annealing the unlabeled complementary DNA with 32P-end-labeled oligonucleotide in 10 mM sodium phosphate (pH 7.0). The mixture was incubated at 90°C for 5 min and slowly cooled to room temperature. The double-stranded DNA products were analyzed on a 10–15% native polyacrylamide gel at 4°C.
Cleavage of DNA oligonucleotides by metal complexes
Cleavage reactions were carried out in a volume of 20 µl containing ∼10 nCi of 5′-γ-32P-labeled DNA, unlabeled DNA (4–5 µM) and 10 mM sodium phosphate (pH 6.96). Metal complexes were added at the indicated concentration and incubated at 25°C for up to 24 h. The reaction was quenched with calf thymus DNA (3–5 µg), 3 M sodium acetate (pH 4.5, 10 µl), and 95% ethanol (900 µl), cooled at –20°C for 30 min, centrifuged (12 000 r.p.m.) at 4°C for 6 min, gently washed with 95% ethanol and lyophilized to dryness.
The sample was resuspended in aqueous 0.7 M piperidine (60 µl), heated at 90°C for 30 min, lyophilized, washed with deionized H2O (20 µl), and lyophilized to dryness. The DNA sample was resuspended in gel-loading buffer (5 µl) containing 0.25% bromophenol blue, 0.25% xylene cyanol FF, and 7 M urea and analyzed on a 20% denaturing polyacrylamide gel (∼7 M urea). The gel was visualized using Kodak BioMax MR-1 film with an intensifying screen. The results were quantified using image programs from UVP Inc. (GelBase/GelBlot™).
Preparation of DNA Maxam–Gilbert G marker
A DNA size marker was prepared using a modified Maxam–Gilbert reaction for G residues (17). Approximately 10 nCi of 32P-labeled oligomer in 20 µl of deionized H2O was incubated at 0–4°C. DMS (<1 µl) was added, the sample mixed thoroughly by vortexing (<1 s) and 2-mercaptoethanol (10 µl) was added with vortexing for 30 s to quench the reaction. The reaction was incubated at 4°C for 6 min. Calf thymus DNA (4 µg) and 3.0 M sodium acetate (pH 6.3, 5 µl) were added and the sample was precipitated with 95% ethanol (1 ml) at –20°C for 30 min. The DNA was collected by centrifugation, gently washed with 95% ethanol, lyophilized to dryness, and treated with piperidine as described above.
MALDI-TOF mass spectroscopy
Purified HIV-27 DNA (20 µM, Fig. 2A) was added to a solution containing Co complex (20 µM) in a volume of 30 µl. After mixing with H2O2 (0.1%), the mixture was incubated for 0–60 min. The reaction was quenched with 3 M NH4OAc (30 µl) and 95% EtOH (1000 µl), cooled at –20°C for 60 min, centrifuged (12 000 r.p.m.) at 4°C for 10 min, and lyophilized to dryness. The sample was treated with piperidine as described above. The pellet was resuspended in deionized water (10 µl) and mixed with the matrix (3-hydroxypicolinic acid; Sigma) for the MALDI-TOF analysis (VOYAGER DE-PRO; Applied Biosystems).
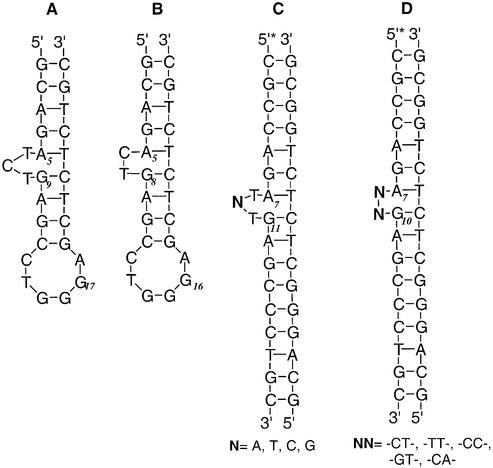
Figure 2.
Structure of DNA substrates.
RESULTS AND DISCUSSION
The geometry of metal–HAPP complexes
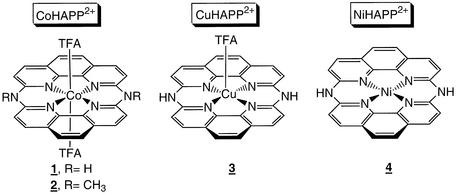
The HAPP ligand was prepared by condensing two molecules of 2,9-dichloro-1,10-phenanthroline at 200°C under flowing ammonia gas (16,18). The yellow product was reacted with CoII, CuII or NiII acetate in TFA/MeOH. The structures of the MII(HAPP)2+ complexes, deduced by X-ray crystallography (Fig. 1), show that each complex has a different coordination geometry. The complexes have two fused 1,10-phenanthroline moieties, with all four pyridinium nitrogens sitting on the same coordination plane, and different numbers of labile axial TFA ligands. NiIIHAPP2+ has a square planar conformation with no axial ligand; CuIIHAPP2+ has a square-pyramidal structure with one axial ligand, and CoIIHAPP2+ has an octahedral structure with two axial TFA ligands. The oxidation state for the metal center of NiHAPP2+ and CuHAPP2+ is the divalent species as indicated by the electronic absorption spectra and redox potential [+0.38 and +0.36 V versus standard calomel electrode (SCE)]. CoII complexes are easily oxidized in the presence of molecular oxygen to form a CoIII species; however, EPR analysis confirmed that the CoHAPP2+ complex is divalent and octahedral with a gav value of 2.005–2.331 in methanol and multiple splitting peaks. The divalent Co complex is stable in air more than a week.
Figure 1.
Chemical structure of macrocyclic metal complexes.
Cleavage of DNA bulges by metal complexes
The CoII, CuII or NiII–HAPP complexes have similar coordination and redox potential, but their reactivity and selectivity for DNA structural perturbations are dramatically different. CoIIHAPP2+ nicks supercoiled (Form I) plasmid DNA catalytically to generate circular DNA (Form II) in the presence of H2O2 (15). The DNA sequence and DNA structure specificity of this reaction was analyzed with 5′-end-labeled DNA fragments and high-resolution polyacrylamide gel electrophoresis. The HIV-27 DNA substrate (Fig. 2A) was a 27mer with the sequence 5′-GCAGATCTGAGCCTGGGAGCTCTCTGC-3′, which forms a double-stranded DNA structure with a three-base bulge and a six-base single-stranded loop (19). This DNA sequence is similar to the sequence of the RNA hairpin from the trans-activation response element (TAR-RNA).
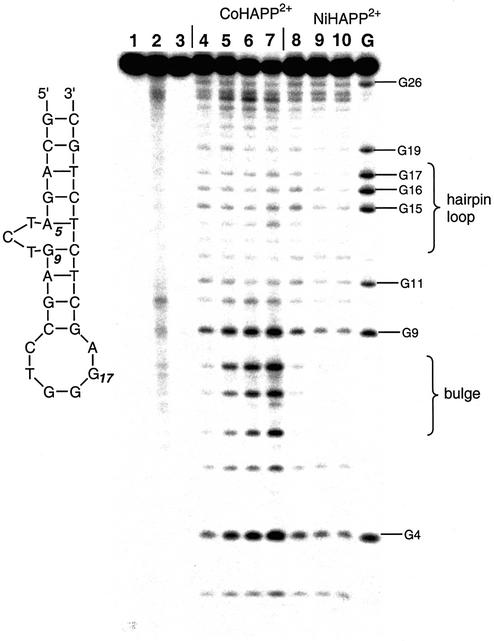
DNA cleavage reactions were performed with metal– HAPP2+ (1–5 µM), 5′-32P-labeled oligonucleotide (4–5 µM) and H2O2 (0.005–0.05%) for 10 min at ambient temperature. If the DNA sample is not treated with piperidine after exposure to CoHAPP2+, nicks are detected predominantly at the bulge (T6, C7 and T8) and very weak nicking is detected at the DNA hairpin loop (C13-A18). The preference for cleavage at the DNA bulge is not a DNA sequence-dependent effect, because both sites have the same 5′-CTG-3′ nucleotide sequence (15). When the DNA sample is treated with piperidine after exposure to CoIIHAPP2+, cleavage is also detected at G4 and G9, due to guanine base oxidation in the presence of external oxidants. DNA was treated with CoCl2 and H2O2 as a positive control; under these conditions, HIV-27 DNA is cleaved relatively non-specifically with more cleavage at guanine residues, as reported previously (20). CuIIHAPP2+ and NiIIHAPP2+ do not cleave the 27mer DNA substrate at the DNA bulge region or hairpin loop in Figure 3. The redox potential of all three HAPP complexes is ∼+0.36 V and Cu2+ and Ni2+ are capable of generating hydroxyl radicals and inducing DNA cleavage in the presence of H2O2 (21,22). Therefore, the geometry of CoHAPP2+ is likely to be important for its ability to selectivity cleave DNA bulges.
Figure 3.
DNA cleavage specificity of metal complexes. Octahedral CoIIHAPP2+ (1, 4 µM), or square planar NiIIHAPP2+ (3, 4 µM) complexes were assayed for DNA cleavage activity using HIV-27 DNA in the presence of H2O2 (0.06%) with (lanes 4–10) or without (lanes 1–3) subsequent piperidine treatment (see Materials and Methods). The structure and DNA sequence of the bulged DNA substrate is shown in Figure 2A. Cleavage products were analyzed on a 20% denaturing polyacrylamide gel (7 M urea). Lane 1, no metal complex; lane 2, complex 1; lane 3, complex 3; lane 4, no metal complex; lanes 5–7, complex 1 at 1, 2, 4 µM; lanes 8–10, complex 3 at 1, 2, 4 µM; lane G, G marker.
The size of the HAPP ring is also crucial for specific recognition of bulged DNA. Another octahedral complex, CoII(cyclam)Cl2, has four amino nitrogens on the same coordination plane as the CoII center and two chloro ligands at axial positions. Thus, it has a geometry similar to CoII(HAPP)(TFA)2 complex. However, CoII(cyclam)2+ cleaves DNA only at accessible guanine residues (23,24), suggesting that ring size is an important factor in determining DNA bulge selectivity.
Sequence dependence of DNA bulge cleavage by CoIIHAPP2+
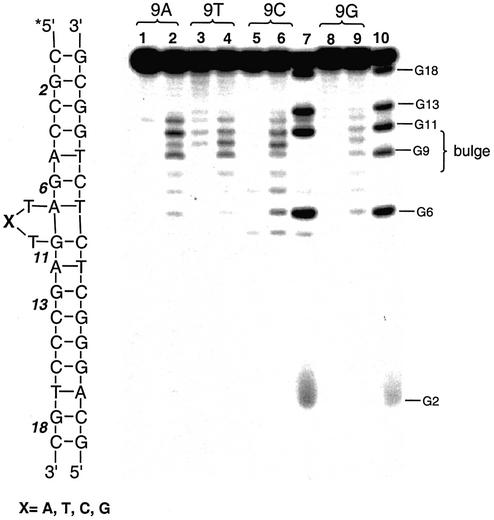
The DNA sequence specificity of CoIIHAPP2+ was examined using DNA substrates with different bulge sequences or a deletion. The C7 position at the bulge of HIV-27 DNA was changed from C to A, T or G (Fig. 2C) and the DNA substrates were tested for reactivity with CoIIHAPP2+. CoIIHAPP2+ preferentially cleaved the bulge site independent of the nucleotide at position C7 (Fig. 4). Similar results were obtained if the DNA substrate had a two-base bulge with the sequence TT, CC, GT or CA (Fig. 2D). The major cleavage sites for these substrates were at the bulge without piperidine treatment and at A7 and G11 after piperidine treatment. However, the DNA sequence did influence the level of reactivity at the bulge, suggesting that DNA structure varies at the bulge in a sequence-dependent manner and this influences the interaction between CoIIHAPP2+ and the DNA bulge.
Figure 4.
DNA sequence-dependence of DNA bulge cleavage by CoIIHAPP2+. DNA cleavage reactions were carried out as described in Figure 3 with 2 µM complex 1 in the presence of H2O2 (0.03%) and 3 µM DNA substrate with variable sequence at position 9 followed by treatment with piperidine. Cleavage products were analyzed on a 20% denaturing polyacrylamide gel (7 M urea). Lanes 1, 3, 5 and 8, DNA control, no complex 1; lanes 2, 4, 6 and 9, with complex 1; lanes 1 and 2, substrate position 9 is A; lanes 3 and 4, position 9 is T; lanes 5–7, position 9 is C; lanes 8–10, position 9 is G; lanes 7 and 10, G marker.
Bulge-specific selectivity of CoHAPP2+ complexes
CoIIHAPP2+ selectively cleaves bulged DNA but not single-stranded DNA. This specificity was demonstrated by comparing cleavage by CoHAPP2+ and H2O2 using a single-stranded 16mer with the sequence 5′-GCCAGATCTG-AGCCTG-3′ and its complementary strand as previously reported (15). Bulge-specific recognition may depend on a hydrogen bond interaction between the bridged amino groups of the HAPP ligand and the nucleic acid substrate. This possibility was investigated using a ligand with methylated amino groups, in which the methyl group blocks the putative H-bond interaction. N-methylated Co(DMHAPP)(TFA)2, 2, was synthesized by methylating the CoHAPP2+ complex with neat CH3I for 7 days at room temperature. The N-methylated Co complex had the same selectivity and reactivity as the unmethylated complex. Thus, it is unlikely that H-bond recognition plays a role in bulge-specific recognition by the CoIIHAPP2+ complex.
It is also possible that the bulge is recognized because the cavity size in the DNA substrate is different at the bulge. The solution structure of a two-base DNA bulge has been analyzed by NMR (10). This structure reveals a triangular prism pocket at the bulge which binds enediyne analogs. When the bulge in HIV-26 DNA (Fig. 2B) involves two bases instead of three, tight binding of the CoIIHAPP2+ complex is observed (15). Unlike bulge structures, many hairpin loop DNA structures have been reported previously. In addition, the pocket of a hairpin loop is usually occupied by unpaired nucleobases which determine its unusual base-pairing conformation. Thus, the hairpin loop may not have enough space to host CoIIHAPP2+ complex.
Mechanism of DNA cleavage by the CoHAPP2+ complex
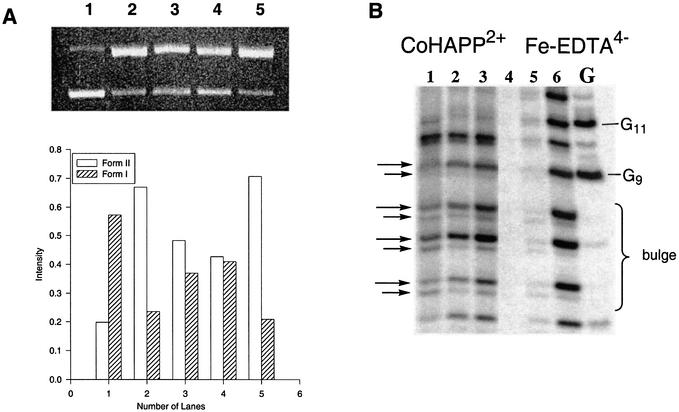
The reactive species involved in DNA cleavage by the CoHAPP2+ complex was analyzed using scavenger reagents as competitors. The hydroxyl radical scavenger mannitol (22) reduced CoIIHAPP2+ nicking of supercoiled DNA and production of relaxed circular DNA (Form II) by >33% (Fig. 5A). However, when superoxide dismutase and D2O were added to a DNA cleavage reaction, production of relaxed circular DNA was not reduced. This result indicates that superoxide and singlet oxygen are probably not involved in DNA cleavage by CoHAPP2+ in the presence of H2O2 and that the hydroxyl radical is likely to play a role in this reaction. The external oxidant H2O2 accelerates DNA cleavage by the metal complex, and when H2O2 is omitted from the reaction, a higher concentration of the CoII complex (>50 µM) and a longer reaction time (>40 min) is required to cleave the same amount of DNA. Because the geometry of the HAPP ligand is similar to porphyrin, it seems possible that photo-induced radicals could also play a role in DNA cleavage by CoIIHAPP2+. This idea was examined by photo-irradiating the CoHAPP2+ complex with a mercury lamp (300 W); however, no DNA cleavage was observed after a 60 min incubation with irradiation.
Figure 5.
Analysis of oxidative cleavage of deoxyribose by CoIIHAPP2+/H2O2. (A) ΦX-174 plasmid DNA (60 µM per nucleotide) was incubated with CoIIHAPP2+ (0.6 µM)/H2O2 (0.01%) in 10 mM sodium phosphate buffer (pH 7) and quenched with mannitol. The cleavage products were analyzed by 1% agarose gel electrophoresis. Lane 1, DNA control, no CoHAPP2+; lanes 2 and 5, CoHAPP2+; lane 3, 25 mM mannitol; lane 4, 50 mM mannitol. (B) CoHAPP2+-induced DNA cleavage products were prepared with or without piperidine treatment. Reactions were analyzed on a 20% denaturing polyacrylamide gel (7 M urea). Fe-EDTA2– DNA cleavage products were prepared as a marker. Lanes 1–3, complex 1, lanes 4 and 5, no complex 1; lane 1, no piperidine or heat; lane 2, heated at 90°C for 30 min; lane 3, complex 1, heated with piperidine; lanes 4, DNA control; lane 5, Fe-EDTA2–, no piperidine; lane 6, Fe-EDTA2–, piperidine; lane G, G marker.
The position at which the deoxyribose moiety of DNA is oxidized during CoIIHAPP2+-catalyzed DNA cleavage was determined in order to better assess the mechanism of the DNA cleavage reaction (25). When the CoHAPP2+-induced DNA cleavage products were treated with or without piperidine, a doublet was detected for each nucleotide unit on high-resolution gels (Fig. 5B), and it is possible that the terminal structure of these cleavage products could reveal information about the mechanism of deoxyribose oxidation. One band of the doublet comigrates with the product of the Maxam–Gilbert G cleavage reaction, which has a 3′-terminal phosphate (17). The other band of the doublet migrates more slowly and is insensitive to alkaline treatment, suggesting that the 3′-terminal group may be larger than phosphate.
The mechanism of sugar oxidation during CoHAPP2+-catalyzed DNA cleavage was also analyzed by treating the DNA cleavage products with several reactive chemicals and examining their effect on high resolution polyacrylamide gels. A similar method was used to study DNA cleavage by the diplatinum complex, Pt2(POP)44– (19,26). 5′- or 3′-32P-labeled HIV-27 DNA was treated with heat, NaBH4, or with CoIIHAPP2+ under anaerobic conditions (25). The electrophoretic mobility of the DNA cleavage products was analyzed using Fe(EDTA)2–-treated DNA as a marker (27). Both bands of the doublet induced by CoIIHAPP2+ and H2O2 are insensitive to NaBH4 and heat; this result was observed with both 5′- and 3′-32P-labeled oligonucleotides. These results indicate that 3′-phosphate-furanone derivatives are not formed during CoHAPP2+-catalyzed DNA cleavage, and that the cleavage mechanism does not utilize a 1′-H abstraction pathway or the 5′-H pathway and 5′-aldehyde nucleotides. Furthermore, the doublet products of CoHAPP2+-catalyzed DNA cleavage comigrate with Fe(EDTA)2–/H2O2 cleavage products (Fig. 5B). These results suggest that CoIIHAPP2+ and H2O2 attacks the ribose 4′-H, which lies in the DNA minor groove.
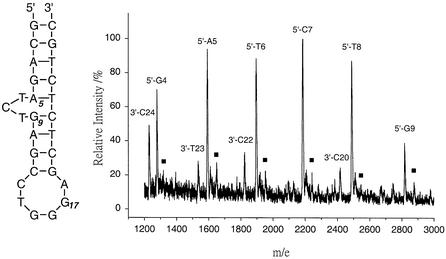
DNA cleavage fragments induced by CoHAPP2+ and H2O2 were also analyzed by MALDI-TOF-MS. MALDI-TOF-MS is an efficient and accurate method to unambiguously characterize the structure of DNA fragments (28–30). MALDI-TOF-MS parameters were carefully optimized to prevent artifactual DNA fragmentation by the laser. The predicted m/e values were calculated from the average molecular weight of the oligonucleotide sequence of predicted cleavage products. MALDI-TOF-MS has higher resolution than gel electrophoresis and showed that the predominant cleavage product has a 3′-terminal phosphate (i.e. 5′-NNN-3′-PO4 where NNN = GCAG, GCAGA, GCAGAT, GCAGATC and GCAGATCT). (Note that products with an m/e value >3000 had very low intensity and are not shown in Fig. 6). The MALDI-TOF-MS data agree well with the gel electrophoresis data, clearly demonstrating that CoHAPP2+ cleaves at bulge positions and the flanking sites A5, T6, C7, T8 and G9 after piperidine treatment.
Figure 6.
MALDI-TOF mass spectrum of CoHAPP2+-induced DNA cleavage products. The CoII complex/H2O2 (0.1%) was incubated with HIV-27 DNA (20 µM) for 10 min and piperidine treatment at 90°C for 30 min. The expected m/e values of 5′- or 3′-cleavage fragments correspond to average [M-H]– values. Cleavage products with a molecular weight 58 m/e units larger than the 5′ fragments are marked with filled squares.
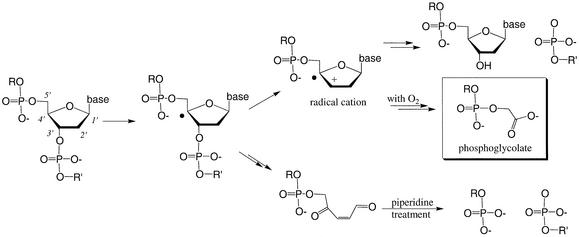
The MALDI-TOF data were used to determine the terminal structure of the DNA cleavage products. The m/e value for several cleavage products was greater than the m/e value of the 3′-terminal PO4– fragment by 58 m/e units (peaks marked with a filled square in Fig. 6). This increase of 58 m/e units is most likely due to a glycolate (-CH2CO2–) moiety (25). Previous studies show that 3′-terminal phosphoglycolate (3′-PO4-CH2CO2–) forms during 4′-H sugar oxidation mediated by the 4′-radical species (30). It is therefore likely that CoIIHAPP2+/H2O2 cleaves DNA by an oxidative mechanism at the 4′-H position of deoxyribose shown in Figure 7.
Figure 7.
Proposed mechanism for CoIIHAPP2+-induced DNA cleavage. Products of 4′-H sugar oxidation pathway during CoIIHAPP2+/H2O2-induced DNA cleavage. The formation of 3′-terminal phosphoglycolate corresponds to formation of a 4′-carbon radical.
SUMMARY
This study demonstrates specific recognition and cleavage of a DNA bulge by a novel octahedral CoII complex. The two axial ligands of CoII(HAPP)(TFA)2 play an important role in bulge specificity. However, an NH hydrogen bond interaction is not likely to play a significant role in bulge specificity. CoHAPP2+-catalyzed cleavage of DNA produces DNA fragments with 3′-terminal phosphoglycolate by a mechanism involving attack at the 4′-H of deoxyribose. These data demonstrate that CoHAPP2+ is a useful reagent for identifying DNA bulges and characterizing perturbations in DNA double-helical structure.
Acknowledgments
ACKNOWLEDGEMENTS
Financial support from Academia Sinica, Tamkang University and the National Science Council of Republic of China is gratefully acknowledged.
REFERENCES
- 1.Streisinger G., Okada,Y., Emrich,J., Newton,J., Tsugita,A., Terzaghi,E. and Inouye,M. (1966) Frameshift mutations and the genetic codes. Cold Spring Harbor Symp. Quant. Biol., 31, 77–84. [DOI] [PubMed] [Google Scholar]
- 2.Streisinger G. and Owen,J. (1985) Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics, 109, 633–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunkel T.A. (1990) Misalignment-mediated DNA synthesis errors. Biochemistry, 29, 8003–8011. [DOI] [PubMed] [Google Scholar]
- 4.Turner D.H. (1992) Bulges in nucleic acids. Curr. Opin. Struct. Biol., 2, 334–337. [Google Scholar]
- 5.Lilley D.M.J. (1995) Kinking of DNA and RNA by base bulges. Proc. Natl Acad. Sci. USA, 92, 7140–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y.-H., Bortner,C.D. and Griffith,J. (1993) RecA binding to bulge- and mismatch-containing DNAs. J. Biol. Chem., 268, 17571–17577. [PubMed] [Google Scholar]
- 7.Zhu J. and Wartell,R.M. (1999) The effect of base sequence on the stability of RNA and DNA single-base bulges. Biochemistry, 38, 15986–15993. [DOI] [PubMed] [Google Scholar]
- 8.Ke S.-H. and Wartell,R.M. (1995) Influence of neighboring base pairs on the stability of single base bulges and base pairs in a DNA fragment. Biochemistry, 34, 4593–4600. [DOI] [PubMed] [Google Scholar]
- 9.Joshua-Tor L., Frolow,F., Appella,E., Hope,H., Rabinovich,D. and Sussman,J.L. (1992) Three-dimensional structures of bulge-containing DNA fragments. J. Mol. Biol., 225, 397–431. [DOI] [PubMed] [Google Scholar]
- 10.Stassinopoulos A., Ji,J., Gao,X. and Goldberg,I.H. (1996) Solution structure of a two-base DNA bulge complexed with an enediyne cleaving analog. Science, 272, 1943–1947. [DOI] [PubMed] [Google Scholar]
- 11.Gollmick F.A., Lorenz,M., Dornberger,U., von Langen,J., Diekmann,S. and Fritzsche,H. (2002) Solution structure of dAATAA and dAAUAA DNA bulges. Nucleic Acids Res., 15, 2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aboul-ela F., Murchie,A.I.H., Homans,S.W. and Lilley,D.M.J. (1993) NMR study of a deoxyoligonucleotide duplex containing a three base bulge. J. Mol. Biol., 229, 173–188. [DOI] [PubMed] [Google Scholar]
- 13.Pratviel G., Bernadou,J. and Meunier,B. (1998) DNA and RNA cleavage by metal complexes. Adv. Inorg. Chem., 45, 251–312. [Google Scholar]
- 14.Armitage B. (1998) Chemical nuclease. Chem. Rev., 98, 1171–1200. [DOI] [PubMed] [Google Scholar]
- 15.Cheng C.C., Kuo,Y.N., Chuang,K.S., Luo,C.F. and Wang,W.J. (1999) A new Co(II) complex as a bulge-specific probe for DNA. Angew. Chem. Int. Ed. Engl., 38, 1255–1257. [DOI] [PubMed] [Google Scholar]
- 16.Chang T.H., Ong,C.W., Chou,Y.M., Chuang,K.S. and Wang,W.J. (1996) Metal ion dependent binding and nuclease activity of hexaazacyclophane. J. Chin. Chem. Soc., 43, 73–75. [Google Scholar]
- 17.Maxam A.M. and Gilbert,W. (1977) A new method for sequencing DNA. Proc. Natl Acad. Sci. USA, 74, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa S. (1977) Preparation of macrocyclic compounds by thermal dimerization of 1,10-phenanthroline derivatives. J. Chem. Soc. Perkin I, 214–216. [Google Scholar]
- 19.Carter P.J., Breiner,K.M. and Thorp,H.H. (1998) The effect of secondary structure on DNA and RNA cleavage by diplatinum(II). Biochemistry, 37, 13736–13743. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K., Inoue,S., Yamazaki,A., Yoshinaga,T. and Kawanishi,S. (1989) Site-specific DNA damage induced by Co(II) and H2O2: role of singlet oxygen. Chem. Res. Toxicol., 2, 234–239. [DOI] [PubMed] [Google Scholar]
- 21.Kasprzak K.S. (1991) The role of oxidative damage in metal carcinogenicity. Chem. Res. Toxicol., 4, 604–615. [DOI] [PubMed] [Google Scholar]
- 22.Sigman D.S., Bruice,T.W., Mazumder,A. and Sutton,C.L. (1993) Targeted chemical nucleases. Acc. Chem. Res., 26, 98. [Google Scholar]
- 23.Burrows C.J. and Rokita,S.E. (1994) Recognition of guanine structure in nucleic acids by nickel complexes. Acc. Chem. Res., 27, 295–301. [Google Scholar]
- 24.Muller J.G., Zheng,P., Rokita,S.E. and Burrows,C.J. (1996) DNA and RNA modification promoted by [Co(H2O)6]Cl2 and KHSO5: guanine selectivity, temperature dependence and mechanism. J. Am. Chem. Soc., 118, 2320–2325. [Google Scholar]
- 25.Pratviel G., Bernadou,J. and Meunier,B. (1995) Carbon-hydrogen bonds of DNA sugar units as targets for chemical nucleases and drugs. Angew. Chem. Int. Ed. Engl., 34, 746–769. [Google Scholar]
- 26.Breiner K.M., Daugherty,M.A., Oas,T.G. and Thorp,H.H. (1995) An anionic diplatinum DNA cleavage agent. J. Am. Chem. Soc., 117, 11673–11679. [Google Scholar]
- 27.Tullius T.D., Dombroski,B.A., Churchill,M.E.A. and Kam,L. (1987) Hydroxyl radical footprinting: a high-resolution method for mapping protein-DNA contacts. Methods Enzymol., 155, 537–558. [DOI] [PubMed] [Google Scholar]
- 28.Grotjahn L., Blocker,H. and Frank,R. (1985) Mass spectrometric sequence analysis of oligonucleotides. Biomed. Mass Spectrom., 12, 514. [Google Scholar]
- 29.McLuckey S.A., Vaidyanathan,G. and Habibi-Goudarzi,S. (1995) Charge vs. neutral nucleobase loss from multiply charged oligonucleotide anions. J. Mass Spectrom., 30, 1222. [Google Scholar]
- 30.Giese B., Beyrich-Graf,X., Erdnabb,P., Giraud,L., Imwinkelried,P., Muller,S.N. and Schwitter,U. (1995) Cleavage of single-stranded 4′-oligonucleotide radicals in the presence of O2. J. Am. Chem. Soc., 117, 6146–6147. [Google Scholar]