Abstract
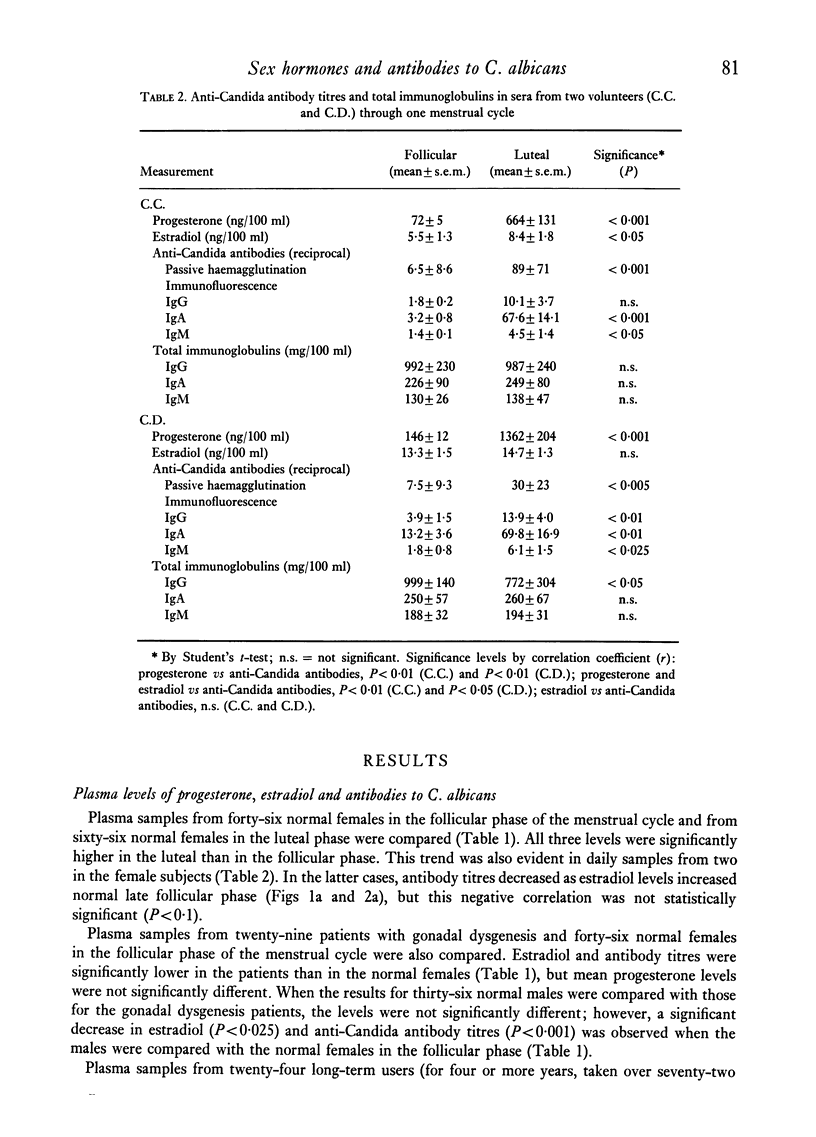
The effect of changes in progesterone (P) and estradiol (E2) on titres of antibodies to Candida albicans was studied by measurement of these three parameters in the following endocrinologically diverse human groups: normal females, gonadal dysgenetics, users of a sequential oral contraceptive (Oracon) and normal males. In females, C. albicans titres (mean +/- s.e.m.) were significantly higher (P less than 0.05) in the luteal (74 +/- 14) than in the follicular phase (34 +/- 19) of the cycle, and there were similar significant increases in P and E2. In the gonadal dysgenetic group (n = 29), with E2 levels comparable with males, the antibody titres were also equivalent to those in normal males (40 +/- 0.5), but were significantly lower than those of normal females in the follicular phase (P less than 0.05). In contrast, Oracon users, with high blood progestin levels, had C. albicans titres (118 +/- 15) significantly higher (P less than 0.001) than those of control subjects during the follicular phase. A significant correlation (P less than 0.05) was observed between P and C. albicans titres (mainly IgA) in randomly selected samples (n = 112) from normal females during the follicular and luteal phases, and in two subjects from whom blood samples were drawn daily for the entire cycle. In the latter, an increase in E2 but not P in the late follicular phase was accompanied by a marked decrease in C. albicans titres. No changes were observed in total immunoglobulin levels or antibodies to SRBC or Herpes virus in response to the marked changes in hormones. These results indicate that the production of antibodies to C. albicans may be specifically influenced by sex steroid hormones, being enhanced by P and E2 at low levels but depressed by E2 at high levels.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. E., Odell W. D., Swerdloff R. S., Hopper K. Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone, and estradiol-17 beta during the menstrual cycle. J Clin Endocrinol Metab. 1972 Feb;34(2):312–318. doi: 10.1210/jcem-34-2-312. [DOI] [PubMed] [Google Scholar]
- BUCK A. A., HASENCLEVER H. F. EPIDEMIOLOGIC STUDIES OF SKIN REACTIONS AND SERUM AGGLUTININS TO CANDIDA ALBICANS IN PREGNANT WOMEN. Am J Hyg. 1963 Sep;78:232–240. doi: 10.1093/oxfordjournals.aje.a120341. [DOI] [PubMed] [Google Scholar]
- Chipperfield E. J., Evans B. A. Effect of local infection and oral contraception on immunoglobulin levels in cervical mucus. Infect Immun. 1975 Feb;11(2):215–221. doi: 10.1128/iai.11.2.215-221.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan B. M., Skinner G. R. Immunoglobulin concentrations in cervical mucus in patients with normal and abnormal cervical cytology. Br J Obstet Gynaecol. 1977 Feb;84(2):129–134. doi: 10.1111/j.1471-0528.1977.tb12539.x. [DOI] [PubMed] [Google Scholar]
- DeVilla G. O., Jr, Roberts K., Wiest W. G., Mikhail G., Flickinger G. A specific radioimmunoassay of plasma progesterone. J Clin Endocrinol Metab. 1972 Sep;35(3):458–460. doi: 10.1210/jcem-35-3-458. [DOI] [PubMed] [Google Scholar]
- Dolan C. T., Stried R. P. Serologic diagnosis of yeast infections. Am J Clin Pathol. 1973 Jan;59(1):49–55. doi: 10.1093/ajcp/59.1.49. [DOI] [PubMed] [Google Scholar]
- Friedman G. D., Collen M. F., Harris L. E., Van Brunt E. E., Davis L. S. Experience in monitoring drug reactions in outpatients. The Kaiser-Permanente Drug Monitoring System. JAMA. 1971 Aug 2;217(5):567–572. [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Lehner T. Immunofluorescent investigation of Candida albicans antibodies in human saliva. Arch Oral Biol. 1965 Nov-Dec;10(6):975–980. doi: 10.1016/0003-9969(65)90091-9. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mathur S., Koistinen G. V., Horger E. O., 3rd, Mahvi T. A., Fudenberg H. H. Humoral immunity in vaginal candidiasis. Infect Immun. 1977 Jan;15(1):287–294. doi: 10.1128/iai.15.1.287-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur S., Koistinen J., Kyong C. U., Horger E. O., 3rd, Virella G., Fudenberg H. H. Antibodies to Candida albicans in IgA-deficient humans. J Infect Dis. 1977 Sep;136(3):436–438. doi: 10.1093/infdis/136.3.436. [DOI] [PubMed] [Google Scholar]
- Orley J., Flórián E. Serologic aspects of pubertal vaginal candidosis. Agglutination and complement fixing tests. Mykosen. 1974 May 1;17(5):107–109. doi: 10.1111/j.1439-0507.1974.tb04280.x. [DOI] [PubMed] [Google Scholar]
- Parker C. R., Jr, Ellegood J. O., Mahesh V. B. Methods for multiple steroid radioimmunoassay. J Steroid Biochem. 1975 Jan;6(1):1–8. doi: 10.1016/0022-4731(75)90021-7. [DOI] [PubMed] [Google Scholar]
- Ross G. T., Cargille C. M., Lipsett M. B., Rayford P. L., Marshall J. R., Strott C. A., Rodbard D. Pituitary and gonadal hormones in women during spontaneous and induced ovulatory cycles. Recent Prog Horm Res. 1970;26:1–62. doi: 10.1016/b978-0-12-571126-5.50005-4. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Sandoe C. P. Sex steroid hormone regulation of IgA and IgG in rat uterine secretions. Nature. 1977 Aug 11;268(5620):534–536. doi: 10.1038/268534a0. [DOI] [PubMed] [Google Scholar]


