Abstract
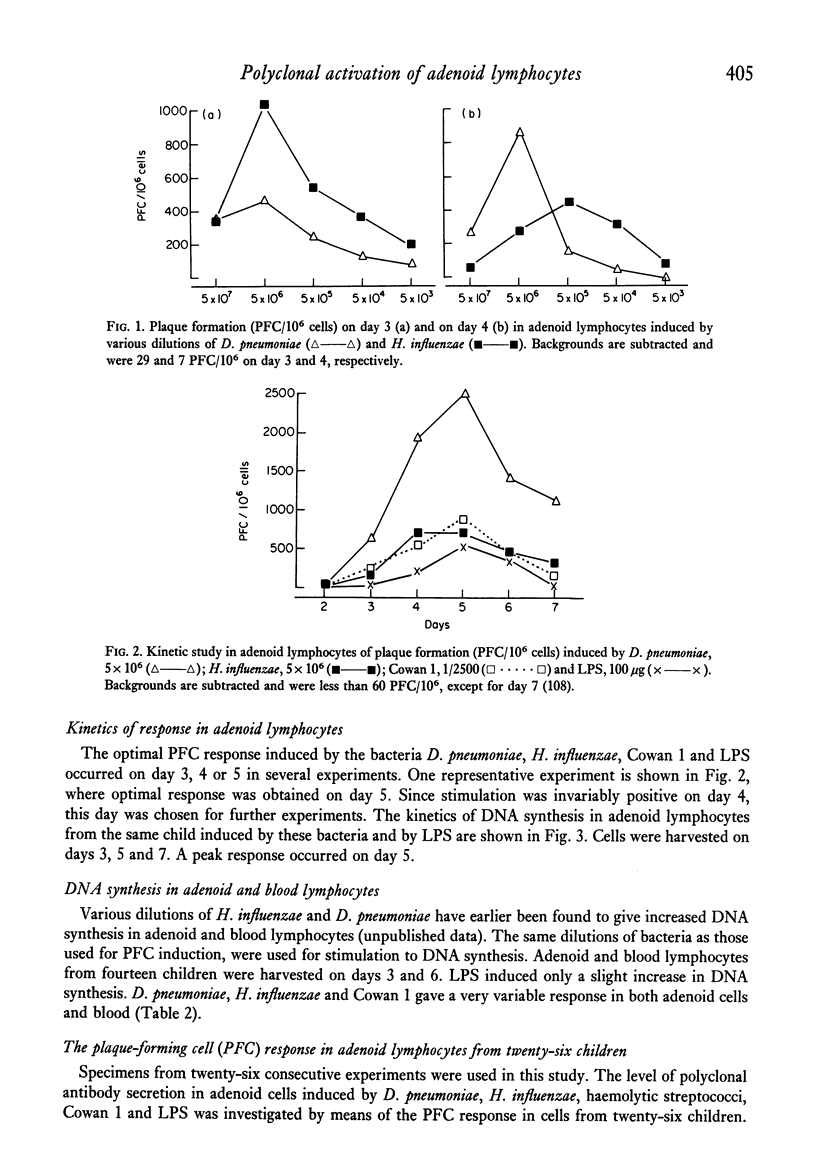
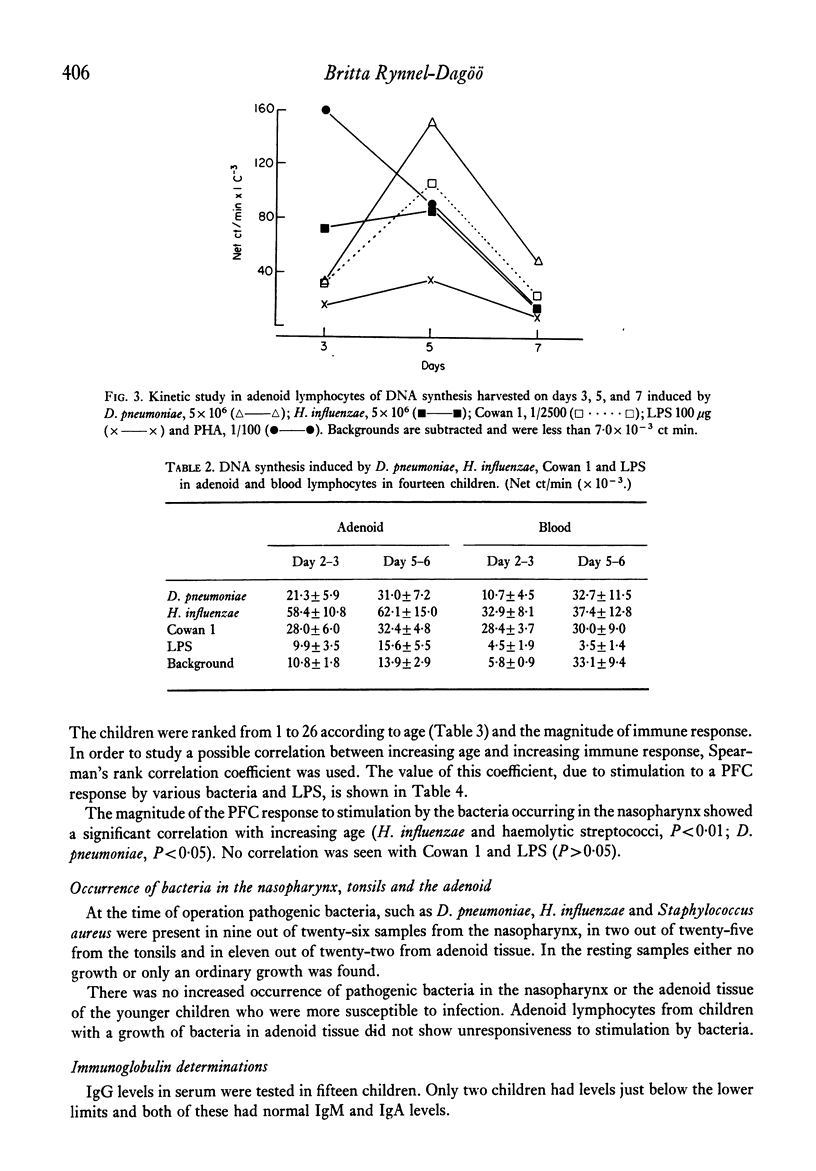
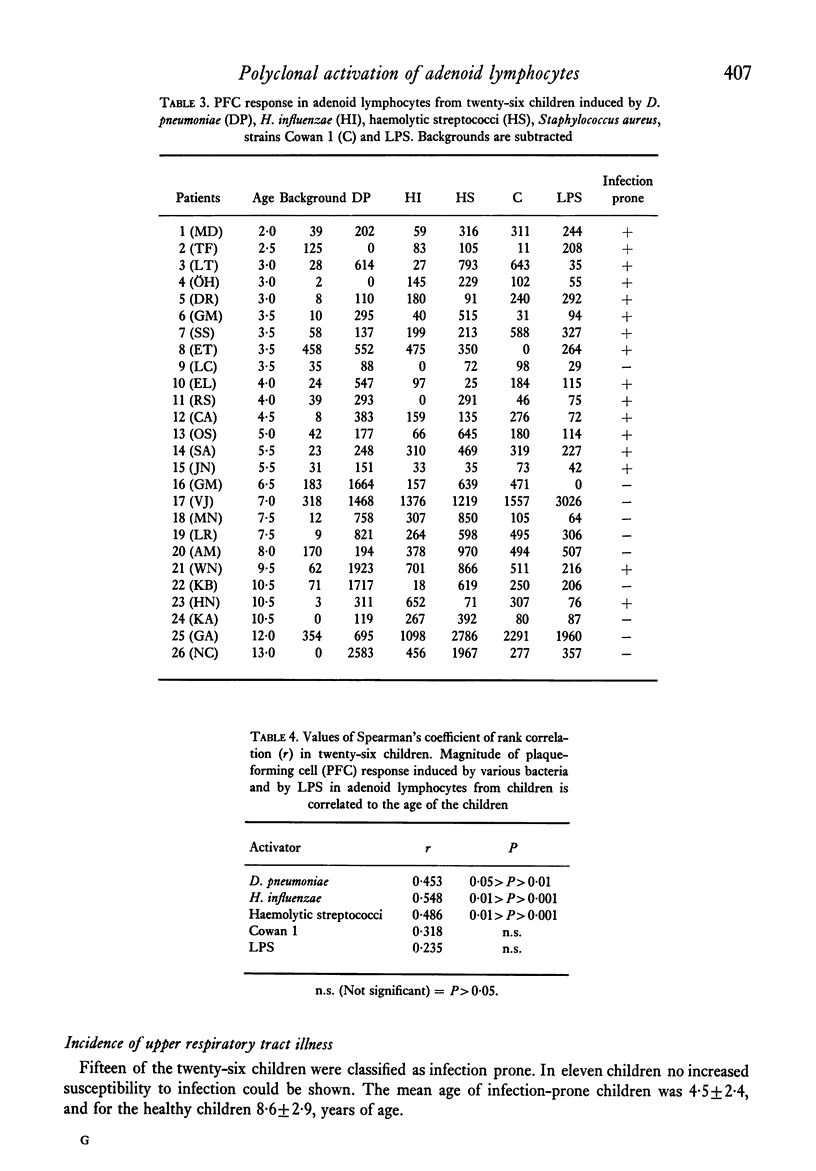
Adenoid lymphocytes from twenty-six children scheduled consecutively for adenoidectomy because of severe nasal obstruction were stimulated in vitro by D. pneumoniae, H. influenzae and haemolytic streptococci group A. All bacteria induced both increased DNA synthesis and polyclonal antibody secretion, tested in a haemolytic plaque assay. The magnitude of the B-cell response was correlated to the age of the children. Thus, cells from older children showed a significantly higher polyclonal response than those from younger children. No case of unresponsiveness to any stimulant was observed. Susceptibility to infection was more pronounced among the younger children, which might reflect some degree of immaturity in the immune system. However, adenoid lymphocytes are immunocompetent cells, contributing to antibody secretion in the response against infection. The role of the receptor for PBA substances on adenoid cells in immune reactions against infectious micro-organisms is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Bullock W. W., Melchers F. Inhibition of mitogenic stimulation of mouse lymphocytes by anti-mouse immunoglobulin antibodies. I. Mode of action. Eur J Immunol. 1974 Nov;4(11):715–722. doi: 10.1002/eji.1830041103. [DOI] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. I. Distribution in different lymphoid organs from different inbred strains of mice at different ages. J Exp Med. 1977 Jun 1;145(6):1511–1519. doi: 10.1084/jem.145.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Melchers F. Maturation of mitogen-activated bone marrow-derived lymphocytes in the absence of proliferation. Eur J Immunol. 1974 Aug;4(8):533–539. doi: 10.1002/eji.1830040803. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Sprent J., Pye J. A receptor for antibody on B lymphocytes. I. Method of detection and functional significance. J Exp Med. 1972 Mar 1;135(3):610–626. doi: 10.1084/jem.135.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld G. Activation of human lymphocyte subpopulations by Mycoplasma pneumoniae. Scand J Immunol. 1977;6(11):1145–1150. doi: 10.1111/j.1365-3083.1977.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Biberfeld G., Gronowicz E. Mycoplasma pneumoniae is a polyclonal B-cell activator. Nature. 1976 May 20;261(5557):238–239. doi: 10.1038/261238a0. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G., Anderson J., Bullock W. W. In vitro activation of mouse lymphocytes in serum-free medium: effect of T and B cell mitogens on proliferation and antibody synthesis. Eur J Immunol. 1973 May;3(5):299–306. doi: 10.1002/eji.1830030509. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Activation of human B lymphocytes. I. Direct plaque-forming cell assay for the measurement of polyclonal activation and antigenic stimulation of human B lymphocytes. J Exp Med. 1976 Sep 1;144(3):674–684. doi: 10.1084/jem.144.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Functional analysis of B cell heterogeneity. Transplant Rev. 1975;24:3–40. doi: 10.1111/j.1600-065x.1975.tb00164.x. [DOI] [PubMed] [Google Scholar]
- HOW M. J., BRIMACOMBE J. S., STACEY M. THE PNEUMOCOCCAL POLYSACCHARIDES. Adv Carbohydr Chem. 1964;19:303–358. doi: 10.1016/s0096-5332(08)60285-4. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamme C., Lundgren K., Märdh P. A. The aetiology of acute otitis media in children. Occurrence of bacteria, L forms of bacteria and mycoplasma in the middle ear exudate. Relationship between bacterial findings in the middle ear exudate, nasopharynx and throat. Scand J Infect Dis. 1971;3(3):217–223. doi: 10.3109/inf.1971.3.issue-3.07. [DOI] [PubMed] [Google Scholar]
- Loda F. A., Collier A. M., Glezen W. P., Strangert K., Clyde W. A., Jr, Denny F. W. Occurrence of Diplococcus pneumoniae in the upper respiratory tract of children. J Pediatr. 1975 Dec;87(6 Pt 2):1087–1093. doi: 10.1016/s0022-3476(75)80120-x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Landwall P. The polyclonal B-cell-activating property of protein A is not due to its interaction with the FC part of immunoglobulin receptors. Scand J Immunol. 1977;6(4):357–366. doi: 10.1111/j.1365-3083.1977.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Nakashima I., Kato N. Non-specific stimulation of immunoglobulin synthesis in mice by capsular polysaccharide of Klebsiella pneumoniae. Immunology. 1974 Aug;27(2):179–193. [PMC free article] [PubMed] [Google Scholar]
- Ringdén O., Rynnel-Dagö B., Waterfield E. M., Möller E., Möller G. Polyclonal antibody secretion in human lymphocytes induced by killed staphylococcal bacteria and by lipopolysaccharide. Scand J Immunol. 1977;6(11):1159–1169. doi: 10.1111/j.1365-3083.1977.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Rynnel-Dagö B., Ahlbom A., Schiratzki H. Effects of adenoidectomy: a controlled two-year follow-up. Ann Otol Rhinol Laryngol. 1978 Mar-Apr;87(2 Pt 1):272–278. doi: 10.1177/000348947808700223. [DOI] [PubMed] [Google Scholar]
- Rynnel-Dagö B. The immunological function of the adenoid. The proportions of T and B cells. Acta Otolaryngol. 1976 Sep-Oct;82(3-4):196–198. doi: 10.3109/00016487609120881. [DOI] [PubMed] [Google Scholar]
- Sloyer J. L., Jr, Howie V. M., Ploussard J. H., Ammann A. J., Austrian R., Johnston R. B., Jr Immune response to acute otitis media in children. I. Serotypes isolated and serum and middle ear fluid antibody in pneumococcal otitis media. Infect Immun. 1974 Jun;9(6):1028–1032. doi: 10.1128/iai.9.6.1028-1032.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim F. A., Williams R. C., Jr Studies on the macroglobulins of human serum. I. Polyclonal immunoglobulin class M (IgM) increase in infectious mononucleosis. N Engl J Med. 1966 Jan 13;274(2):61–67. doi: 10.1056/NEJM196601132740202. [DOI] [PubMed] [Google Scholar]
- ZAMENHOF S., LEIDY G., FITZGERALD P. L., ALEXANDER H. E., CHARGAFF E. Polyribophosphate, the type-specific substance of Hemophilus influenzae, type b. J Biol Chem. 1953 Aug;203(2):695–704. [PubMed] [Google Scholar]


