Abstract
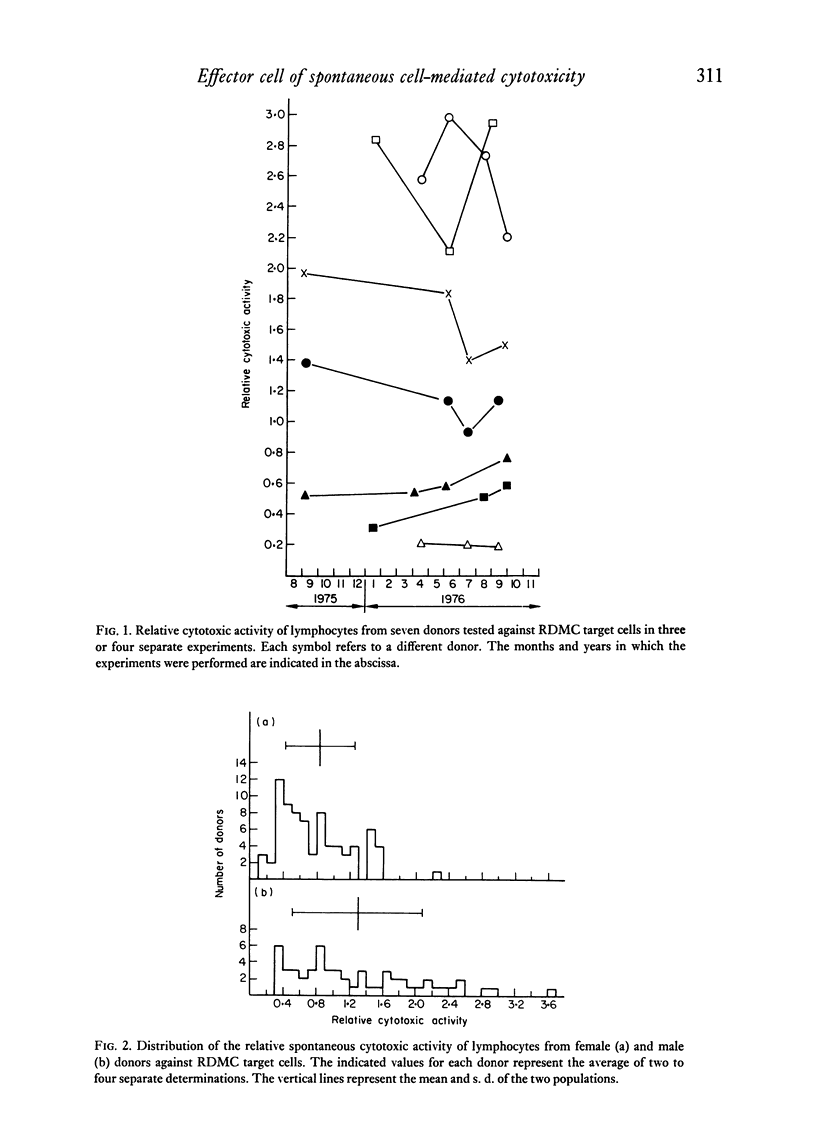
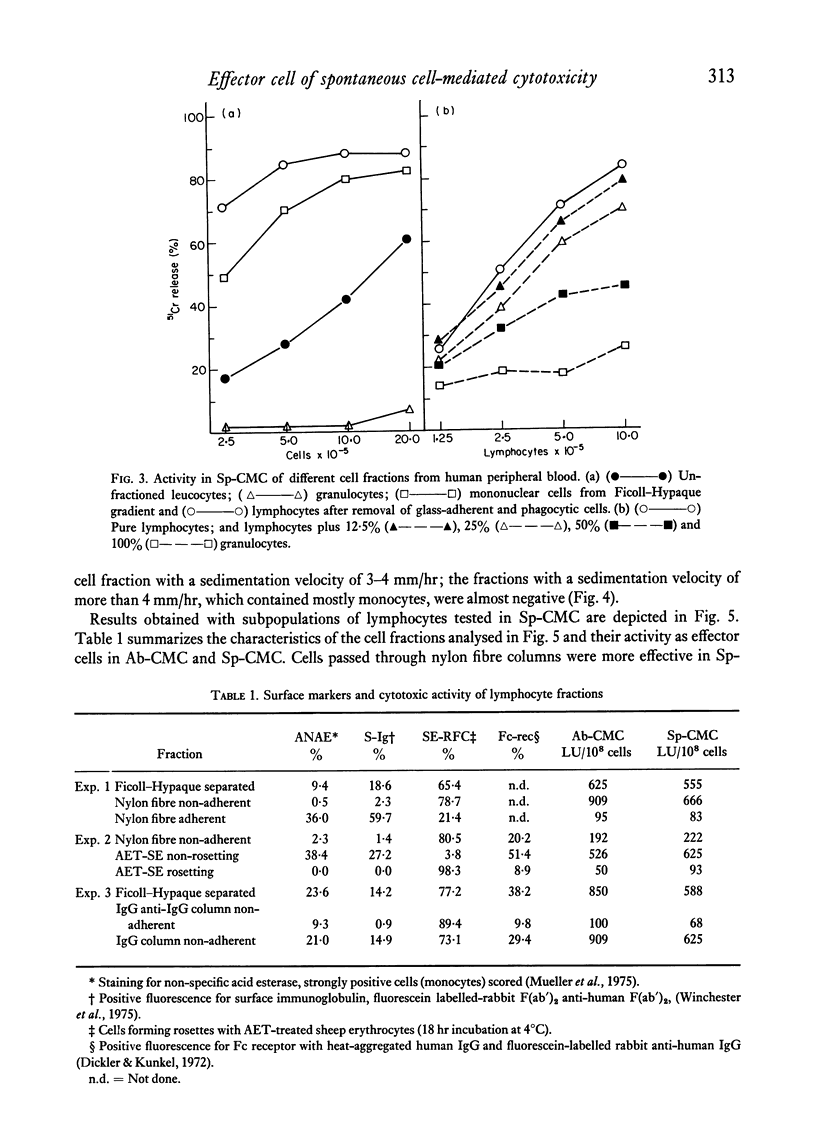
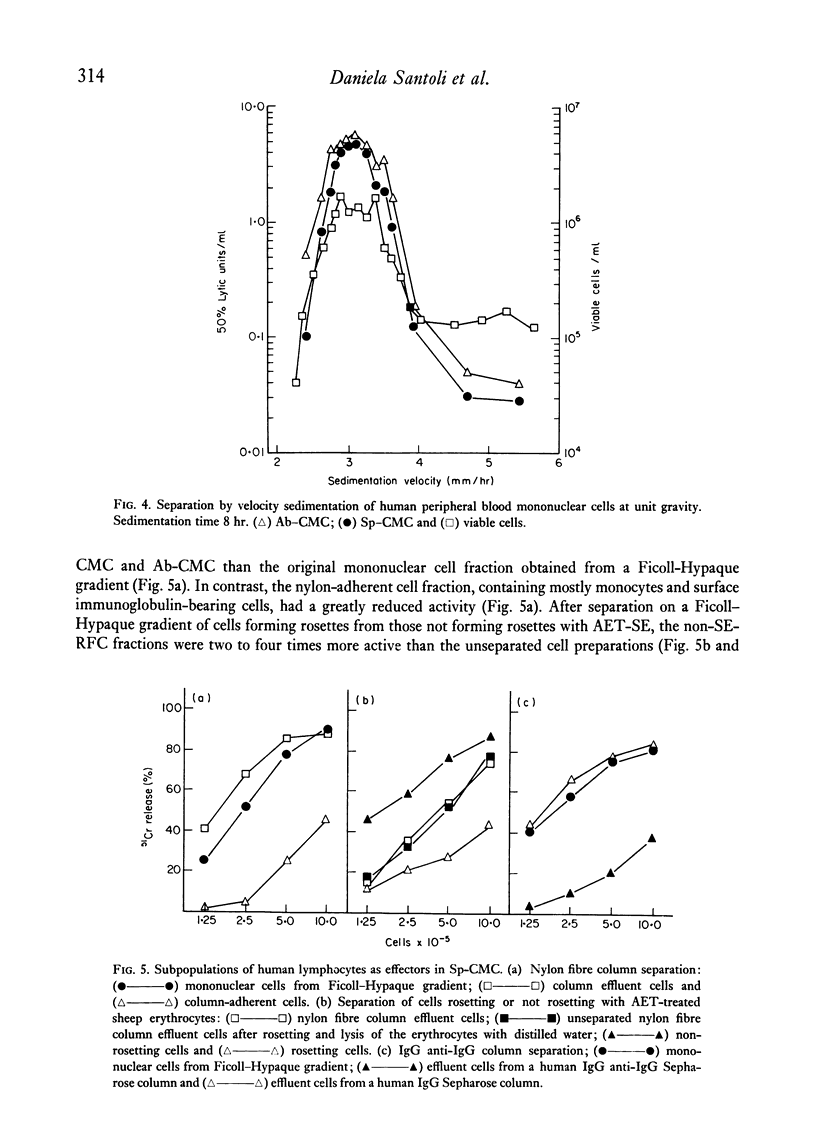
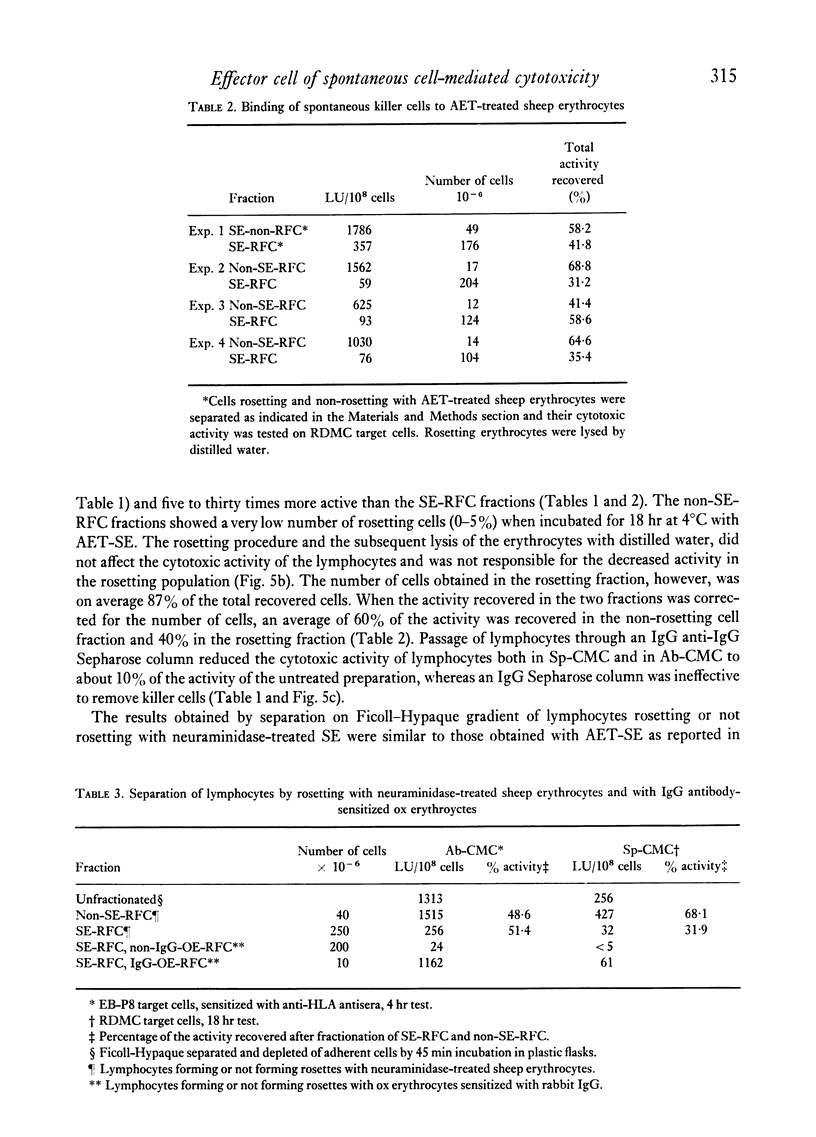
When lymphocytes from healthy donors were tested as effector cells, the cytotoxic activities observed in spontaneous and in antibody-dependent cell-mediated cytotoxicity were positively correlated. However, with lymphocyte preparations obtained from renal patients, a dissociation between the two activities was occasionally observed. Human natural killer cells are lymphocytes, with receptors for the Fc fragment of IgG molecules, but with no surface immunoglobulin. Their cytotoxicity is reduced by the presence of granulocytes or monocytes. After separation of rosetting and non-rosetting cells with AET- (2-aminoethylisothiouronium bromide hydrobromide) or neuraminidase-treated sheep erythrocytes, the majority of the activity was recovered in the non-rosetting fraction, but a portion of it was present consistently in the rosetting cell fraction. Cells in the latter fraction also displayed receptors for the Fc fragment of immunoglobulin G.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira D., Takasugi M. Loss of specific natural cell-mediated cytotoxicity with absorption of natural antibodies from serum. Int J Cancer. 1977 Jun 15;19(6):747–755. doi: 10.1002/ijc.2910190603. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Hersey P., MacLennan I. Two unconventional mechanisms for immunological enhancement. Transplant Proc. 1972 Jun;4(2):277–280. [PubMed] [Google Scholar]
- Jondal M., Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975 Apr 15;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kay H. D., Bonnard G. D., West W. H., Herberman R. B. A functional comparison of human Fc-receptor-bearing lymphocytes active in natural cytotoxicity and antibody-dependent cellular cytotoxicity. J Immunol. 1977 Jun;118(6):2058–2066. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kovithavongs T., Hottman W. C., Dossetor J. B. Effector cell activity in antibody-mediated cell-dependent immune lympholysis. I. Normal individuals. J Immunol. 1974 Oct;113(4):1178–1183. [PubMed] [Google Scholar]
- Lundgren G., Zukoski C. F., Möller G. Differential effects of human granulocytes and lymphocytes on human fibroblasts in vitro. Clin Exp Immunol. 1968 Oct;3(8):817–836. [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Mueller J., Brun del Re G., Buerki H., Keller H. U., Hess M. W., Cottier H. Nonspecific acid esterase activity: a criterion for differentiation of T and B lymphocytes in mouse lymph nodes. Eur J Immunol. 1975 Apr;5(4):270–274. doi: 10.1002/eji.1830050411. [DOI] [PubMed] [Google Scholar]
- Nunn M. E., Djeu J. Y., Glaser M., Lavrin D. H., Herberman R. B. Natural cytotoxic reactivity of rat lymphocytes against syngeneic Gross virus-induced lymphoma. J Natl Cancer Inst. 1976 Feb;56(2):393–399. doi: 10.1093/jnci/56.2.393. [DOI] [PubMed] [Google Scholar]
- Pape G. R., Troye M., Perlmann P. Characterization of cytolytic effector cells in peripheral blood of healthy individuals and cancer patients. I. Surface markers and K cell activity after separation of B cells and lymphocytes and Fc-receptors by column fractionation. J Immunol. 1977 Jun;118(6):1919–1924. [PubMed] [Google Scholar]
- Pape G. R., Troye M., Perlmann P. Characterization of cytolytic effector cells in peripheral blood of healthy individuals and cancer patients. II. Cytotoxicity to allogeneic or autochthonous tumor cells in tissue culture. J Immunol. 1977 Jun;118(6):1925–1930. [PubMed] [Google Scholar]
- Peter H. H., Pavie-Fischer J., Fridman W. H., Aubert C., Cesarini J. P., Roubin R., Kourilsky F. M. Cell-mediate cytotoxicity in vitro of human lymphocytes against a tissue culture melanoma cell line (igr3). J Immunol. 1975 Aug;115(2):539–548. [PubMed] [Google Scholar]
- Petrányi G. G., Benczur M., Onody C. E., Hollán S. R. Letter: HL-A 3,7 and lymphocyte cytotoxic activity. Lancet. 1974 Apr 20;1(7860):736–736. doi: 10.1016/s0140-6736(74)92943-2. [DOI] [PubMed] [Google Scholar]
- Relations of HL-A and Rh systems to immune reactivity. Joint report of the results of 'HL-A and immune response' workshop, Budapest 1972. Vox Sang. 1974;26(5):470–482. [PubMed] [Google Scholar]
- Shellam G. R., Hogg N. Gross-virus-induced lymphoma in the rat. IV. Cytotoxic cells in normal rats. Int J Cancer. 1977 Feb 15;19(2):212–224. doi: 10.1002/ijc.2910190211. [DOI] [PubMed] [Google Scholar]
- Takasugi M., Mickey M. R., Terasaki P. I. Reactivity of lymphocytes from normal persons on cultured tumor cells. Cancer Res. 1973 Nov;33(11):2898–2902. [PubMed] [Google Scholar]
- Trinchieri G., De Marchi M., Mayr W., Savi M., Ceppellini R. Lymphocyte antibody lymphocytolytic interaction (LALI) with special emphasis on HL-A. Transplant Proc. 1973 Dec;5(4):1631–1649. [PubMed] [Google Scholar]
- Trinchieri G., Santoli D., Knowles B. B. Tumour cell lines induce interferon in human lymphocytes. Nature. 1977 Dec 15;270(5638):611–613. doi: 10.1038/270611a0. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D., Koprowski H. Spontaneous cell-mediated cytotoxicity in humans: role of interferon and immunoglobulins. J Immunol. 1978 Jun;120(6):1849–1855. [PubMed] [Google Scholar]
- Trinchieri G., Santoli D., Zmijewski C. M., Koprowski H. Functional correlation between antibody-dependent and spontaneous cytotoxic activity of human lymphocytes and possibility of an HLA-related control. Transplant Proc. 1977 Mar;9(1):881–884. [PubMed] [Google Scholar]
- Winchester R. J., Fu S. M., Hoffman T., Kunkel H. G. IgG on lymphocyte surfaces; technical problems and the significance of a third cell population. J Immunol. 1975 Apr;114(4):1210–1212. [PubMed] [Google Scholar]


