Abstract
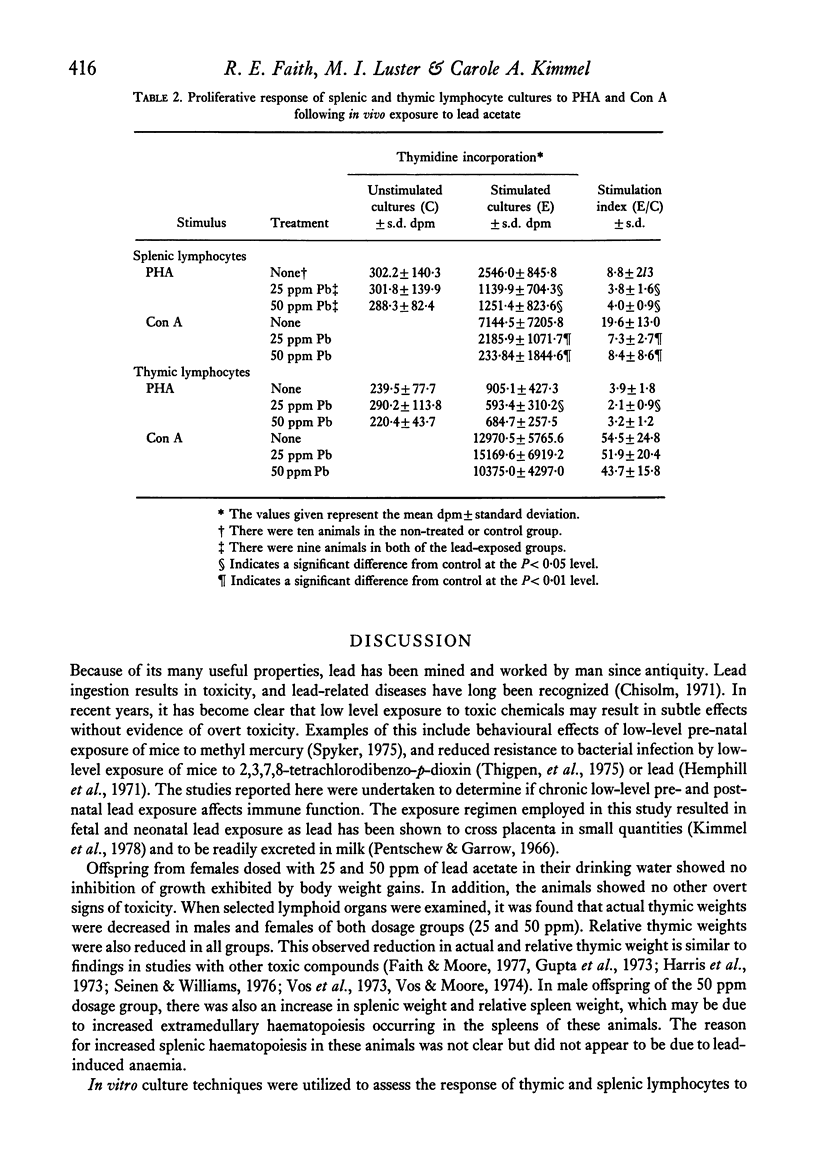
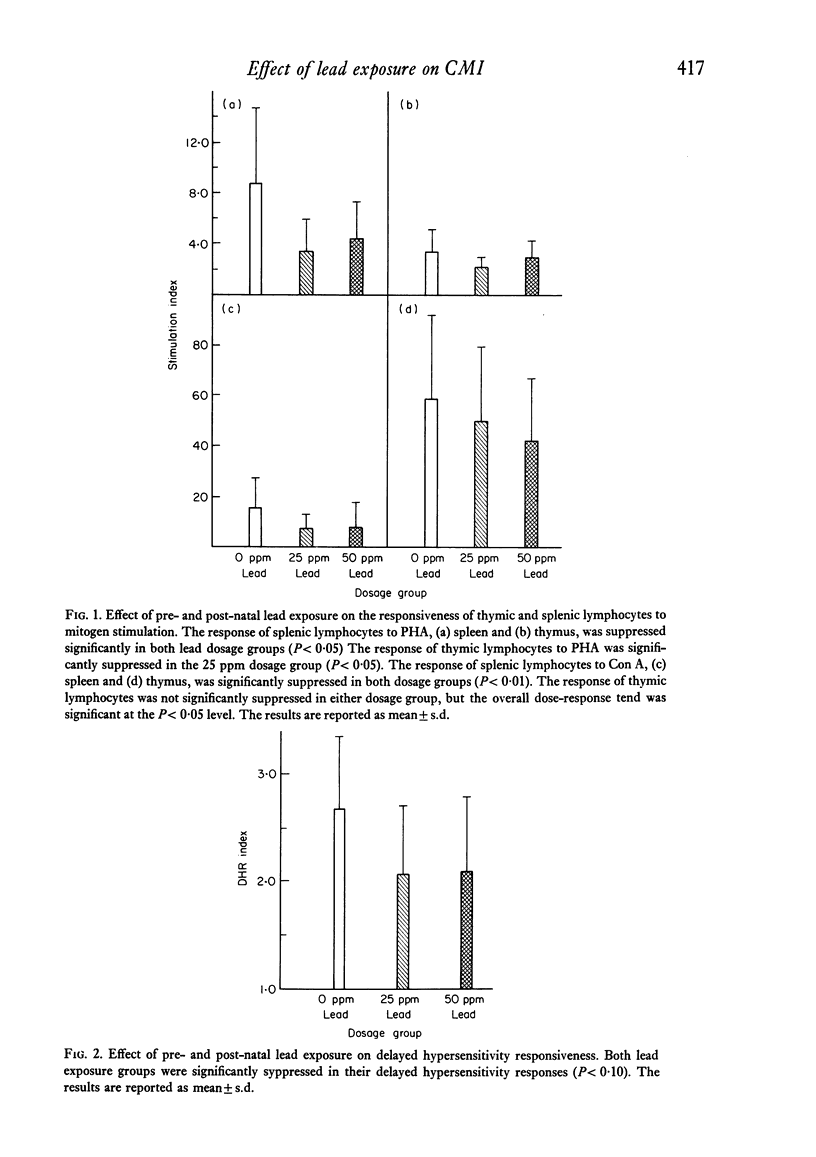
Studies were performed to investigate the effects of chronic, low level pre- and post-natal lead exposure on cell-mediated immune function in rats. Weanling female rats were exposed to lead (as lead acetate) in their drinking water at 0, 25, and 50 ppm for 7 weeks. At the end of 7 weeks they were mated with untreated males and continued on the same dosage throughout gestation and lactation. The offspring of these females were weaned at 21 days of age and continued on the same lead exposure regimen as their mothers. These offspring were used in immune surveillance procedures between 35 and 45 days of age. Lead exposure at the levels employed had no statistically significant effect on growth and did not result in overt signs of toxicity. Thymic weights were significantly decreased in both males and females of the two lead dosage groups. Furthermore, lead exposure resulted in suppression of responsiveness of lymphocytes to mitogen stimulation and in reduced delayed hypersensitivity responsiveness. Results indicate that chronic low-level lead exposure causes suppression of cell-mediated immune function.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantor H., Weissman I. Development and function of subpopulations of thymocytes and T lymphocytes. Prog Allergy. 1976;20:1–64. [PubMed] [Google Scholar]
- Caprio R. J., Margulis H. L., Joselow M. M. Lead absorption in children and its relationship to urban traffic densities. Arch Environ Health. 1974 Apr;28(4):195–197. doi: 10.1080/00039896.1974.10666468. [DOI] [PubMed] [Google Scholar]
- Faith R. E., Moore J. A. Impairment of thymus-dependent immune functions by exposure of the developing immune system to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). J Toxicol Environ Health. 1977 Oct;3(3):451–464. doi: 10.1080/15287397709529578. [DOI] [PubMed] [Google Scholar]
- Friend M., Trainer D. O. Polychlorinated biphenyl: interaction with duck hepatitis virus. Science. 1970 Dec 18;170(3964):1314–1316. doi: 10.1126/science.170.3964.1314. [DOI] [PubMed] [Google Scholar]
- GESNER B. M., GINSBURG V. EFFECT OF GLYCOSIDASES ON THE FATE OF TRANSFUSED LYMPHOCYTES. Proc Natl Acad Sci U S A. 1964 Sep;52:750–755. doi: 10.1073/pnas.52.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B. N., Vos J. G., Moore J. A., Zinkl J. G., Bullock B. C. Pathologic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals. Environ Health Perspect. 1973 Sep;5:125–140. doi: 10.1289/ehp.7305125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. W., Moore J. A., Vos J. G., Gupta B. N. General biological effects of TCDD in laboratory animals. Environ Health Perspect. 1973 Sep;5:101–109. doi: 10.1289/ehp.7305101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill F. E., Kaeberle M. L., Buck W. B. Lead suppression of mouse resistance to Salmonella typhimurium. Science. 1971 Jun 4;172(3987):1031–1032. doi: 10.1126/science.172.3987.1031. [DOI] [PubMed] [Google Scholar]
- Jones R. H., Williams R. L., Jones A. M., Eveland W. C. Effects of heavy metal on the immune response. Preliminary findings for cadmium in rats. Proc Soc Exp Biol Med. 1971 Sep;137(4):1231–1236. doi: 10.3181/00379727-137-35762. [DOI] [PubMed] [Google Scholar]
- Koller L. D. Immunosuppression produced by lead, cadmium, and mercury. Am J Vet Res. 1973 Nov;34(11):1457–1458. [PubMed] [Google Scholar]
- Koller L. D., Kovacic S. Decreased antibody formation in mice exposed to lead. Nature. 1974 Jul 12;250(462):148–150. doi: 10.1038/250148a0. [DOI] [PubMed] [Google Scholar]
- Koller L. D., Roan J. G. Effects of lead and cadmium on mouse peritoneal macrophages. J Reticuloendothel Soc. 1977 Jan;21(1):7–12. [PubMed] [Google Scholar]
- Koller L. D., Thigpen J. E. Biphenyl-exposed rabbits. Am J Vet Res. 1973 Dec;34(12):1605–1606. [PubMed] [Google Scholar]
- Lefford M. J. The measurement of tuberculin hypersensitivity in rats. Int Arch Allergy Appl Immunol. 1974;47(4):570–585. doi: 10.1159/000231251. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Asofsky R. In vivo effects of antithymocyte serum on the homing patterns and graft-versus-host reactivity of murine splenic lymphocytes. Cell Immunol. 1974 Mar 30;11(1-3):19–29. doi: 10.1016/0008-8749(74)90003-3. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis P., Ford W. L. Comparative migration of B- and T-Lymphocytes in the rat spleen and lymph nodes. Cell Immunol. 1976 May;23(2):254–267. doi: 10.1016/0008-8749(76)90191-x. [DOI] [PubMed] [Google Scholar]
- Ohi G., Fukuda M., Seto H., Yagyu H. Effect of methylmercury on humoral immune responses in mice under conditions simulated to practical situations. Bull Environ Contam Toxicol. 1976 Feb;15(2):175–180. doi: 10.1007/BF01685157. [DOI] [PubMed] [Google Scholar]
- Pentschew A., Garro F. Lead encephalo-myelopathy of the suckling rat and its implications on the porphyrinopathic nervous diseases. With special reference to the permeability disorders of the nervous system's capillaries. Acta Neuropathol. 1966 Jun 1;6(3):266–278. doi: 10.1007/BF00687857. [DOI] [PubMed] [Google Scholar]
- Seinen W., Willems M. I. Toxicity of organotin compounds. I. Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed di-n-octyltindichloride. Toxicol Appl Pharmacol. 1976 Jan;35(1):63–75. doi: 10.1016/0041-008x(76)90111-3. [DOI] [PubMed] [Google Scholar]
- Spyker J. M. Assessing the impact of low level chemicals on development: behavioral and latent effects. Fed Proc. 1975 Aug;34(9):1835–1844. [PubMed] [Google Scholar]
- Street J. C., Sharma R. P. Alteration of induced cellular and humoral immune responses by pesticides and chemicals of environmental concern: quantitative studies of immunosuppression by DDT, aroclor 1254, carbaryl, carbofuran, and methylparathion. Toxicol Appl Pharmacol. 1975 Jun;32(3):587–602. doi: 10.1016/0041-008x(75)90123-4. [DOI] [PubMed] [Google Scholar]
- Thigpen J. E., Faith R. E., McConnell E. E., Moore J. A. Increased susceptibility to bacterial infection as a sequela of exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Infect Immun. 1975 Dec;12(6):1319–1324. doi: 10.1128/iai.12.6.1319-1324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo R. A., Di Luzio N. R., Loose L. D., Hoffman E. Reticuloendothelial and hepatic functional alterations following lead acetate administration. Exp Mol Pathol. 1972 Oct;17(2):145–158. doi: 10.1016/0014-4800(72)90064-0. [DOI] [PubMed] [Google Scholar]
- Verschuuren H. G., Ruitenberg E. J., Peetoom F., Helleman P. W., van Esch G. J. Influence of triphenyltim acetate on lymphatic tissue and immune responses in guinea pigs. Toxicol Appl Pharmacol. 1970 Mar;16(2):400–410. doi: 10.1016/0041-008x(70)90011-6. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Moore J. A. Suppression of cellular immunity in rats and mice by maternal treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Int Arch Allergy Appl Immunol. 1974;47(5):777–794. doi: 10.1159/000231268. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Moore J. A., Zinkl J. G. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the immune system of laboratory animals. Environ Health Perspect. 1973 Sep;5:149–162. doi: 10.1289/ehp.7305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. G., de Roij T. Immunosuppressive activity of a polychlorinated diphenyl preparation on the humoral immune response in guinea pigs. Toxicol Appl Pharmacol. 1972 Apr;21(4):549–555. doi: 10.1016/0041-008x(72)90011-7. [DOI] [PubMed] [Google Scholar]
- Weissman I. L. Thymus cell migration. J Exp Med. 1967 Aug 1;126(2):291–304. doi: 10.1084/jem.126.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J., Gesner B. M. Lymphocytes: circulation altered by trypsin. Science. 1968 Jul 12;161(3837):176–178. doi: 10.1126/science.161.3837.176. [DOI] [PubMed] [Google Scholar]


