Abstract
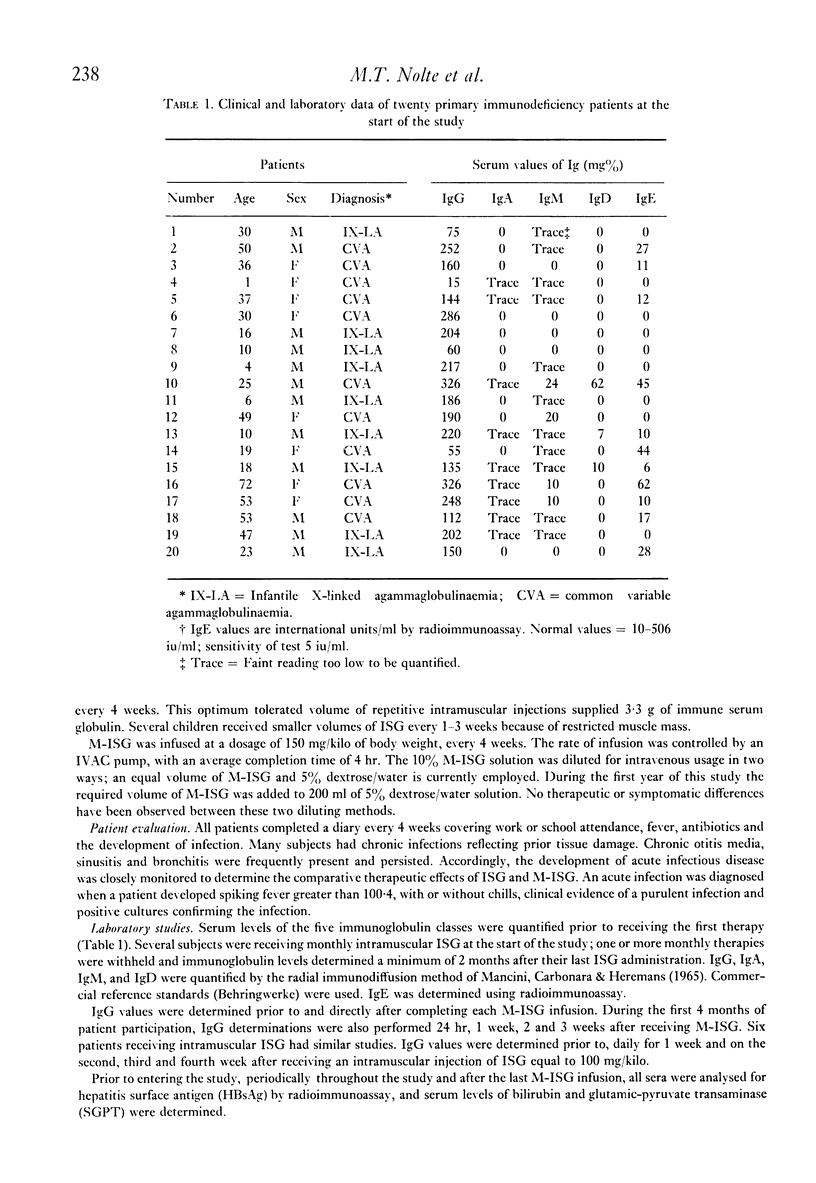
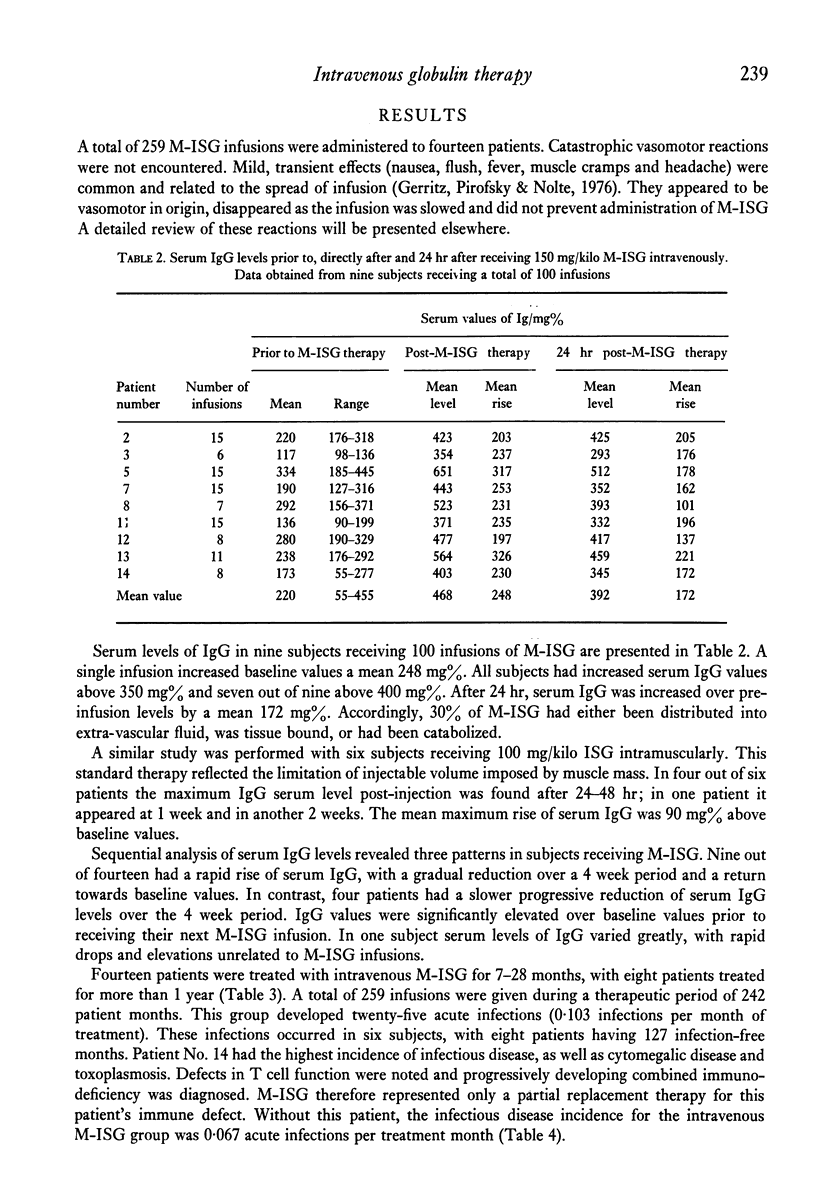
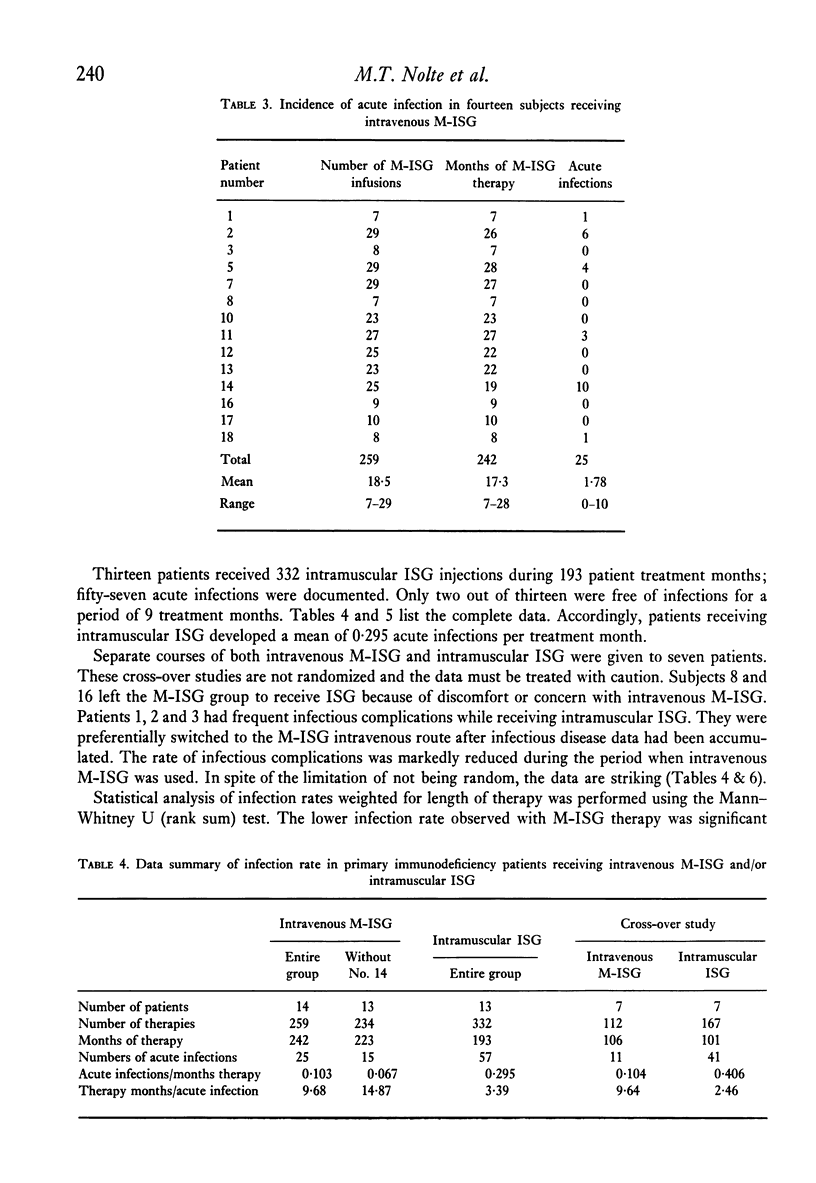
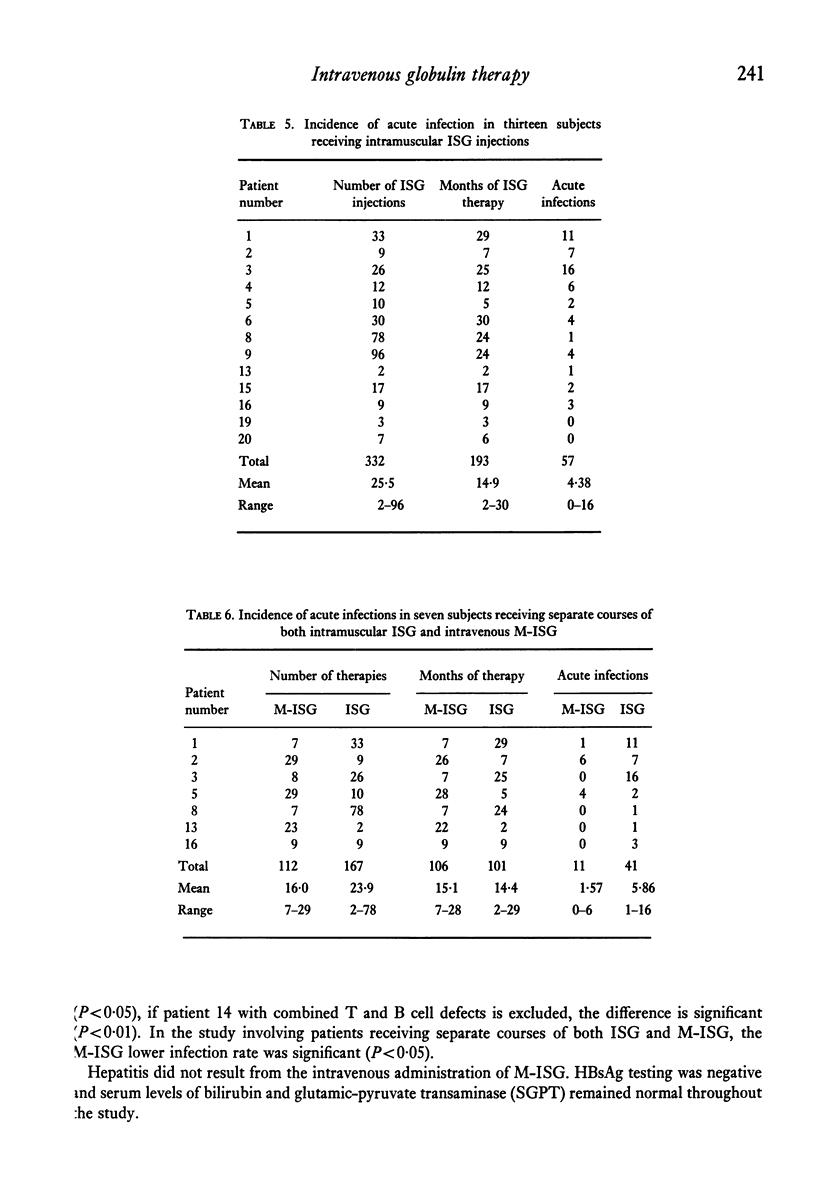
Twenty patients with antibody deficiency were treated at random with either intramuscular immune serum globulin (ISG) or intravenous modified immune serum globulin (M-ISG). Fourteen patients received of 259 M-ISG infusions during 242 months of treatment. Catastrophic vasomotor reactions were not observed. A single dose of 150 mg/kilo M-ISG increased serum IgG values a mean 248 mg%. Intravenous M-ISG therapy was effective in reducing the incidence of acute infections. Subjects receiving M-ISG developed 0.103 acute infections per month of treatment. Patients injected with ISG had 0.295 acute infections per month of treatment. Seven subjects had separate courses of both intravenous M-ISG and intramuscular ISG. Acute infections per month of treatment for M-ISG and ISG were 0.104 and 0.406, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARANDUN S., KISTLER P., JEUNET F., ISLIKER H. Intravenous administration of human gamma-globulin. Vox Sang. 1962;7:157–174. doi: 10.1111/j.1423-0410.1962.tb03240.x. [DOI] [PubMed] [Google Scholar]
- DOMZ C. A., DICKSON D. R. The agammaglobulinemias; relations and implications. Am J Med. 1957 Dec;23(6):917–927. doi: 10.1016/0002-9343(57)90302-9. [DOI] [PubMed] [Google Scholar]
- GITLIN D., JANEWAY C. A. Agammaglobulinemia, congenital, acquired and transient forms. Prog Hematol. 1956;1:318–329. [PubMed] [Google Scholar]
- Hitzig W. H., Müntener U. Conventional immunoglobulin therapy. Birth Defects Orig Artic Ser. 1975;11(1):339–342. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- Janeway C. A., Merler E., Rosen F. S., Salmon S., Crain J. D. Metabolism of gamma globulin fragments in normal and agammaglobulinemic persons. N Engl J Med. 1968 Apr 25;278(17):919–923. doi: 10.1056/NEJM196804252781701. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Rosen F. S. The gamma globulins. IV. Therapeutic uses of gamma globulin. N Engl J Med. 1966 Oct 13;275(15):826–831. doi: 10.1056/NEJM196610132751508. [DOI] [PubMed] [Google Scholar]
- Koblet H., Barandun S., Diggelmann H. Turnover of standard-gammaglobulin, pH-4-gammaglobulin and pepsin desaggregated gammaglobulin and clinical implications. Vox Sang. 1967 Jul;13(1):93–102. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Merler E., Rosen F. S., Salmon S., Crain J. D., Janeway C. A. Studies with intravenous gamma globulin. Vox Sang. 1967 Jul;13(1):102–103. [PubMed] [Google Scholar]
- Painter R. H., Walcroft M. J., Weber J. C. The efficacy of fragmented immune serum globulin in passive immunization. Can J Biochem. 1966 Mar;44(3):381–387. doi: 10.1139/o66-045. [DOI] [PubMed] [Google Scholar]
- SCHULTZE H. E., SCHWICK G. [On new possibilities of intravenous gamma globulin administration]. Dtsch Med Wochenschr. 1962 Aug 24;87:1643–passim. doi: 10.1055/s-0028-1113997. [DOI] [PubMed] [Google Scholar]
- Sgouris J. T. The preparation of plasmin treated immune serum globulin for intravenous use. Vox Sang. 1967 Jul;13(1):71–84. [PubMed] [Google Scholar]
- Smith G. N., Griffiths B., Mollison D., Mollison P. L. Uptake of IgG after intramuscular and subcutaneous injection. Lancet. 1972 Jun 3;1(7762):1208–1212. doi: 10.1016/s0140-6736(72)90926-9. [DOI] [PubMed] [Google Scholar]
- Stephan W., Hainski M., Payne J. H., Ordonez G. A., Shanbrom E. Undegraded -globulin for intravenous therapy. A new preparation of immune serum globulin for intravenous administration. Vox Sang. 1971 May;20(5):469–478. doi: 10.1111/j.1423-0410.1971.tb01827.x. [DOI] [PubMed] [Google Scholar]
- Wells J. V., Penny R. Survival studies on a commercial preparation of intravenous human gammaglobulin labelled with 131-I. Australas Ann Med. 1969 Aug;18(3):271–276. doi: 10.1111/imj.1969.18.3.271. [DOI] [PubMed] [Google Scholar]


