Abstract
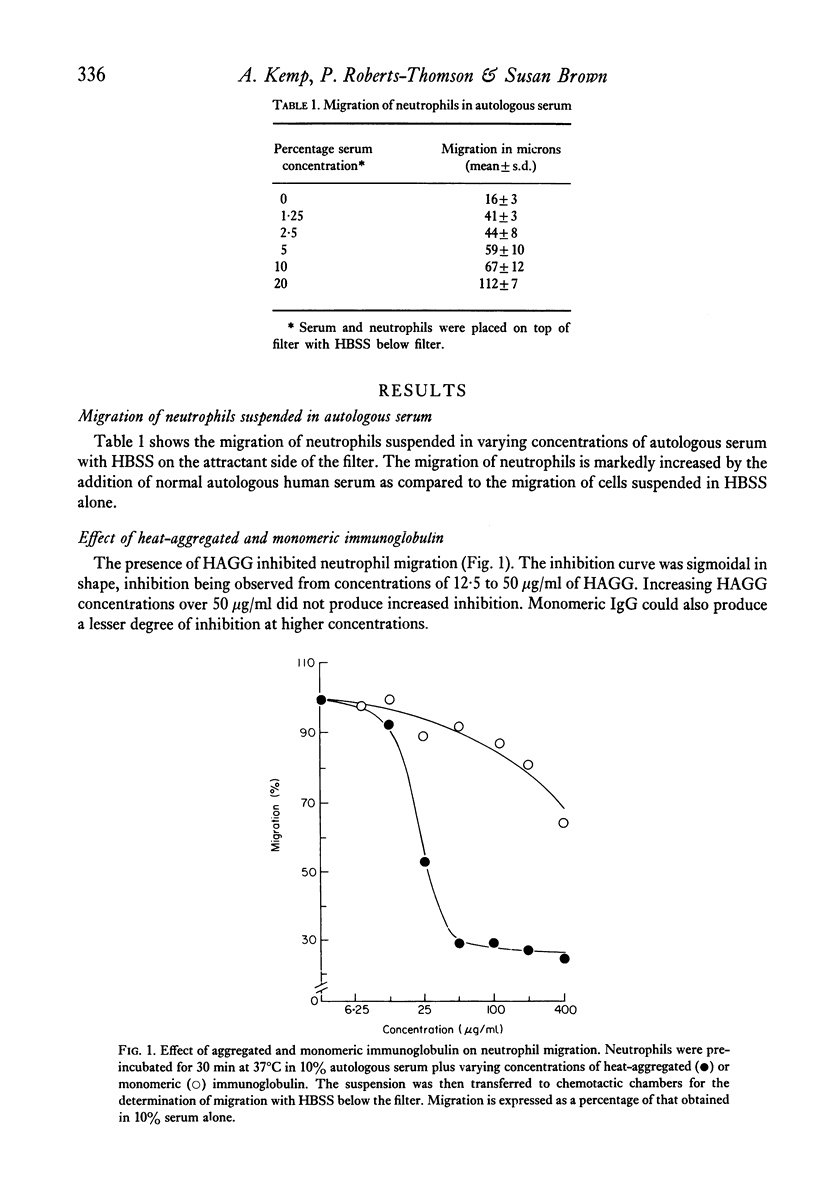
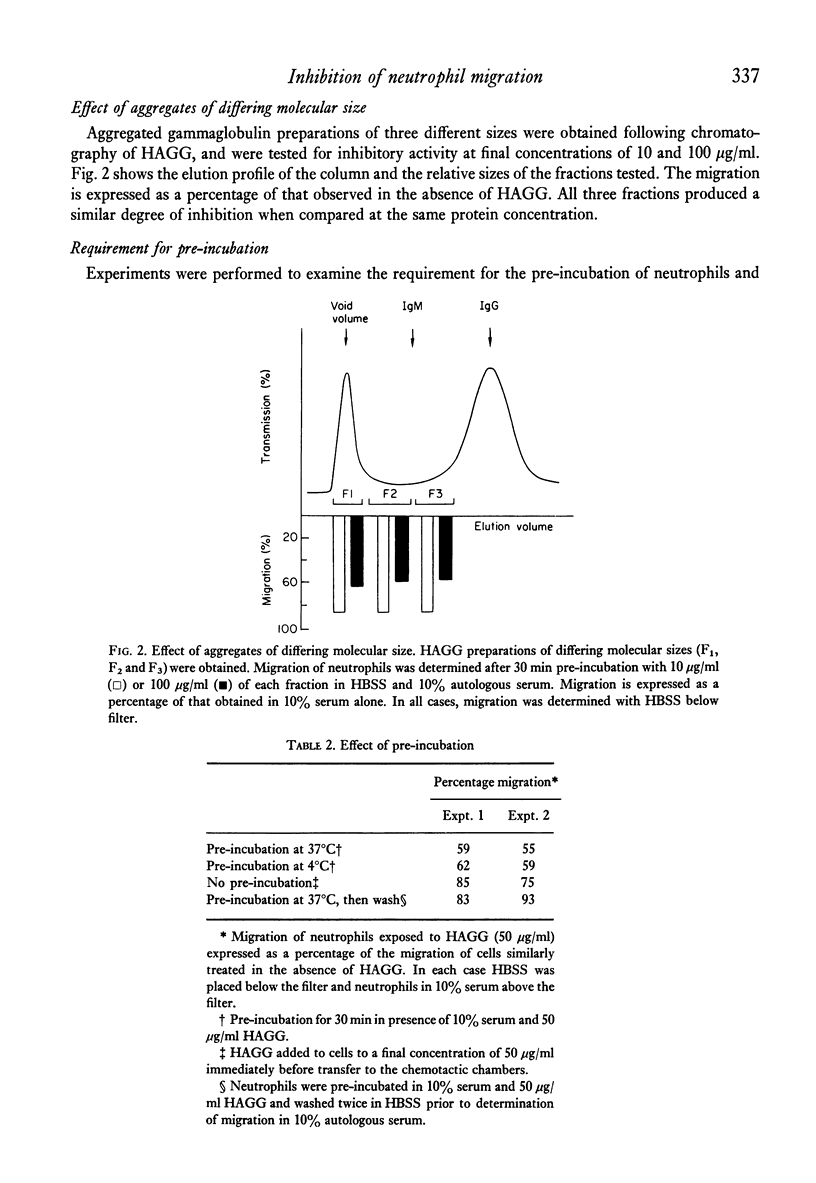
Heat-aggregated human gammaglobulin has been shown to inhibit the random migration of human neutrophils in serum-containing medium. This inhibition was not due to metabolic exhaustion or deactivation of the cells, since migration in the presence of aggregated gammaglobulin and casein as a chemotactic stimulus was not inhibited. The inhibition of migration was not mediated by a negative chemotactic gradient produced as a result of complement activation, and could be demonstrated in complement-depleted serum. Sera obtained from patients with rheumatoid arthritis with evidence of circulating immune complexes were able to significantly inhibit neutrophil migration, indicating that this phenomenon may be a useful means for the detection of circulating immune complexes. It is suggested that aggregated gammaglobulin or immune complexes can inhibit the chemokinetic effect of serum on neutrophils by a reversible interaction with the neutrophil surface, and that this inhibition could contribute to the accumulation of neutrophils at sites of immune complex deposition in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Henson P. M. Membrane receptors on neutrophils. Immunol Commun. 1976;5(9):757–774. doi: 10.3109/08820137609047618. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Wilkinson P. C., Abercrombie M., Becker E. L., Hirsch J. G., Miller M. E., Scottramsey W., Zigmond S. H. A proposal for the definition of terms related to locomotion of leucocytes and other cells. Clin Exp Immunol. 1977 Mar;27(3):377–380. [PMC free article] [PubMed] [Google Scholar]
- Laster C. E., Gleich G. J. Chemotaxis of eosinophils and neutrophils by aggregated immunoglobulins. J Allergy Clin Immunol. 1971 Nov;48(5):297–304. doi: 10.1016/0091-6749(71)90031-5. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Weigle W. O., Spiegelberg H. L. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975 Feb;55(2):368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Howard A., Gotch F. M., Quie P. G. Effector activating determinants on IgG. I. The distribution and factors influencing the display of complement, neutrophil and cytotoxic B-cell determinants on human IgG sub-classes. Immunology. 1973 Sep;25(3):459–469. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. G., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with rheumatoid arthritis. J Clin Invest. 1971 Dec;50(12):2541–2549. doi: 10.1172/JCI106754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Snyderman R., Phillips J. K., Mergenhagen S. E. Biological activity of complement in vivo. Role of C5 in the accumulation of polymorphonuclear leukocytes in inflammatory exudates. J Exp Med. 1971 Nov 1;134(5):1131–1143. doi: 10.1084/jem.134.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Phillips J., Mergenhagen S. E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and Guinea pig serum treated with immune complexes: evidence for c5a as the major chemotactic factor. Infect Immun. 1970 Jun;1(6):521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Wagner T., Abraham G., Baum J. The roles of IgG, IgM rheumatoid factor, and their complexes in the induction of polymorphonuclear leukocyte chemotactic factor from complement. J Clin Invest. 1974 Jun;53(6):1503–1511. doi: 10.1172/JCI107700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]


