Abstract
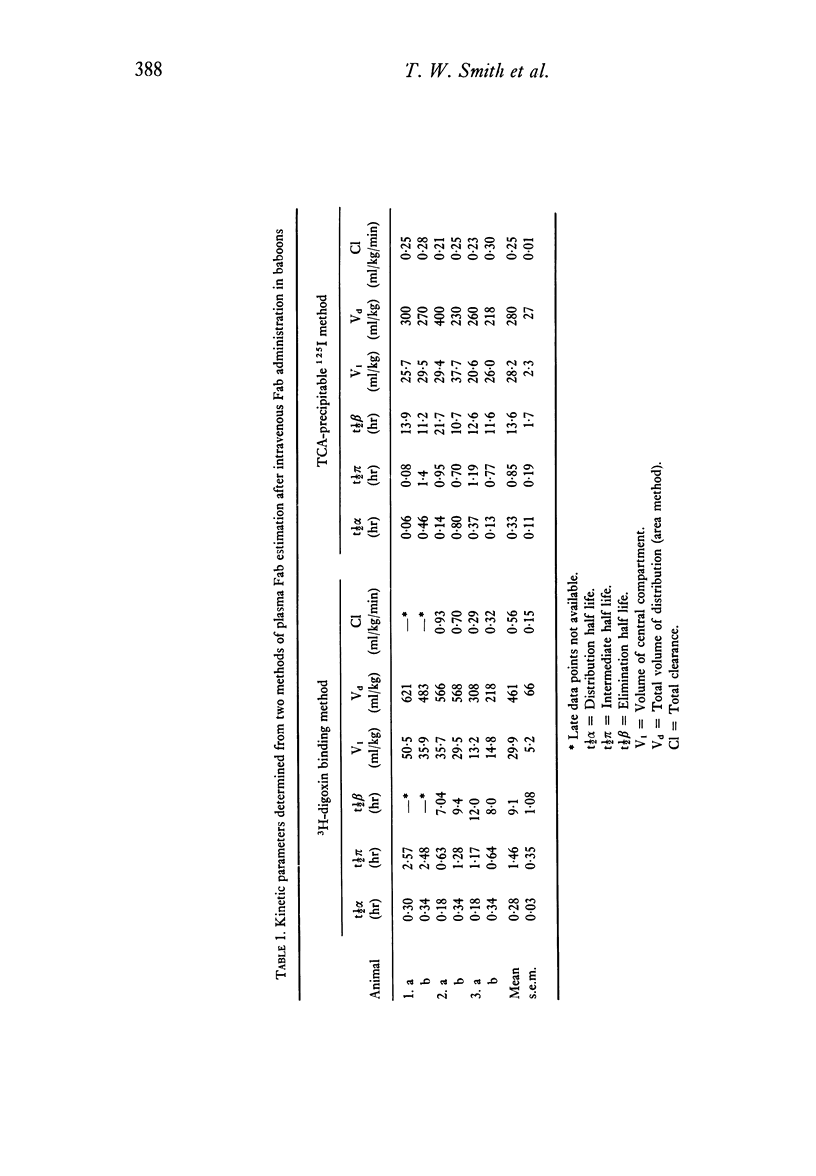
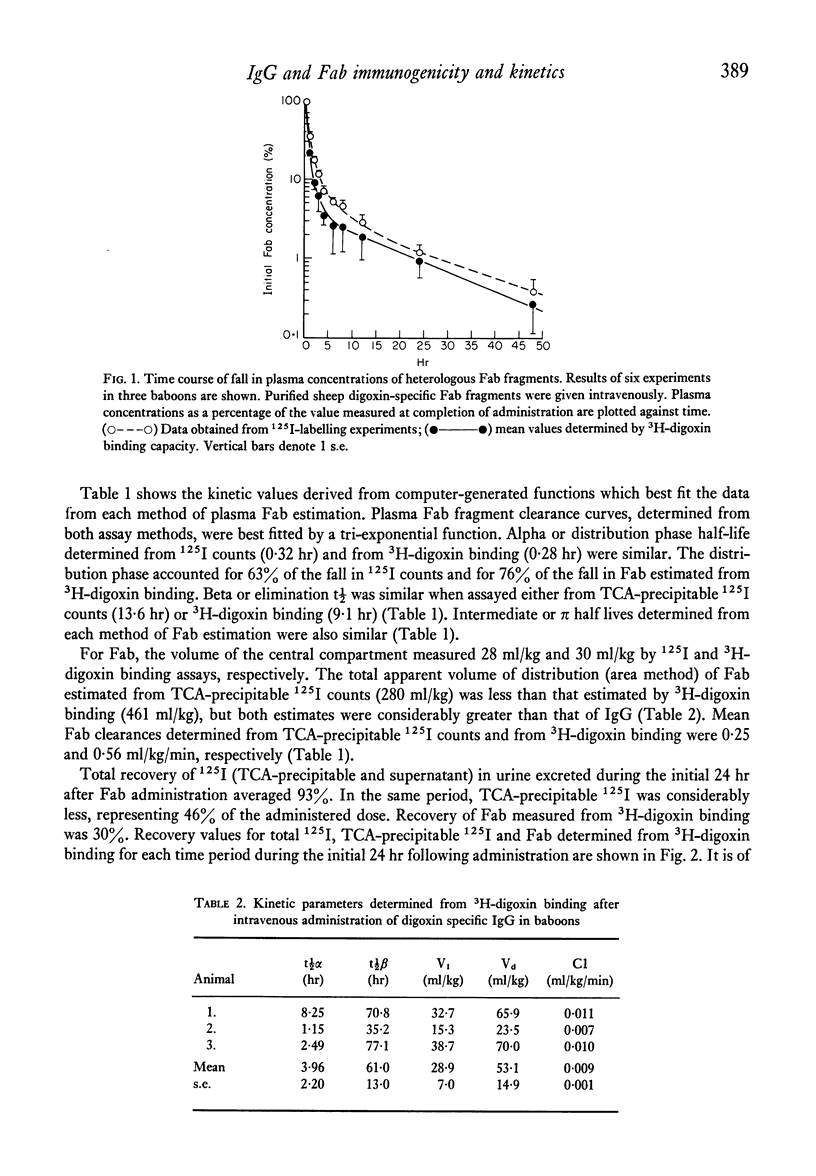
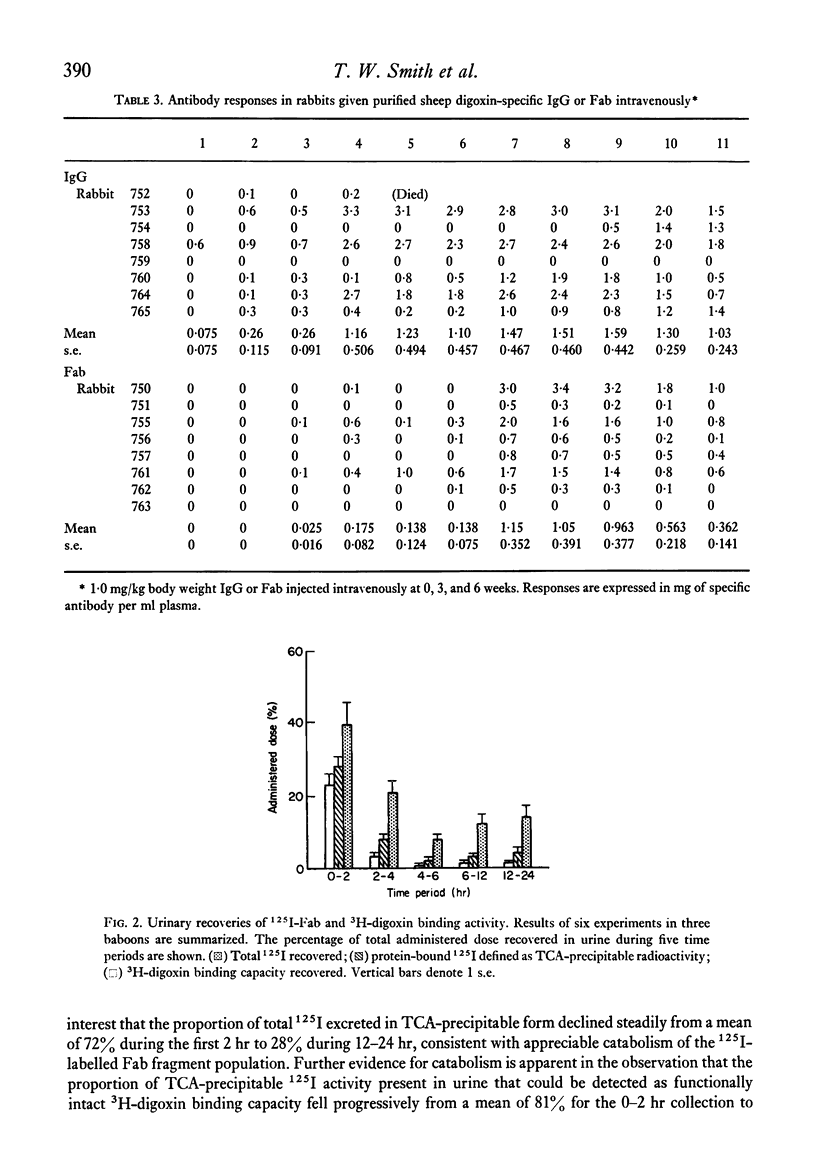
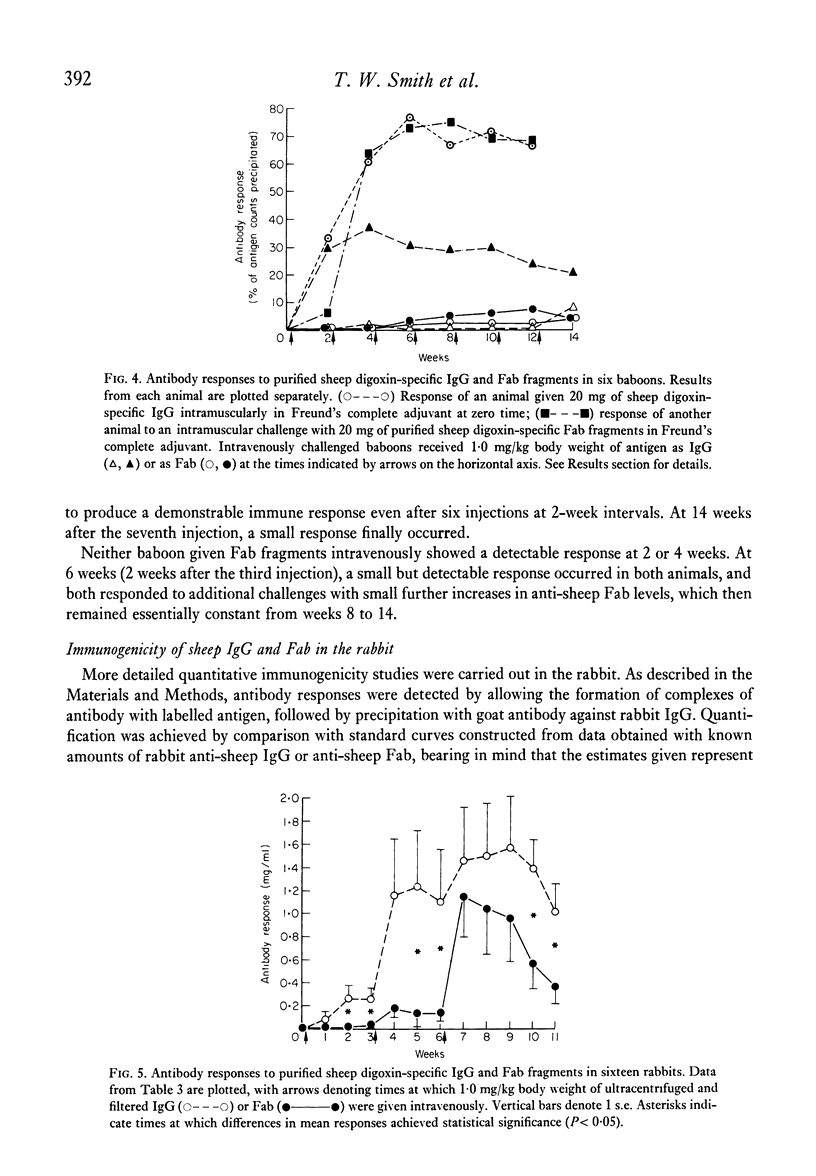
To evaluate the relative merits of purified IgG and Fab preparations of defined specificity for potential clinical use, immunogenicity studies were carried out in baboon and rabbit experimental models. Distribution and elimination kinetics of purified sheep digoxin-specific IgG and Fab fragments were also studied following intravenous administration to baboons. Serial plasma and urine Fab concentrations were determined from trichloroacetic acid-precipitable 125I counts from pre-labelled preparations and also by measurement of the antibody's functional 3H-digoxin binding capacity. Results were compared with data obtained from IgG by 3H-digoxin binding. Kinetic data analysed by computer-fitted functions demonstrated that plasma Fab disappearance was best described by a tri-exponential function, whereas a bi-exponential function best described the IgG data. Initial distribution half-life (t 1/2) of Fab (0.28-0.32 hr) was considerably shorter than that of IgG (4.0 hr) and contributed a greater proportion of the total fall in plasma level over 24 hr. Fab elimination t 1/2 (9-13 hr) was also shorter than IgG (61 hr), but appreciably longer than earlier estimates in rabbits, guinea-pigs, rats and mice. The total volume of distribution of Fab was 8.7 times greater than that of IgG measured by the same method. Over the first 24 hr after administration 30-45% of administered Fab was recoverable in active form in urine, while 93% of total administered 125I counts from 125I-Fab preparations (bound and free) could be recovered. Less than 1% of administered IgG binding activity was recovered in urine during the initial 24 hr. The relative immunogenicities of sheep digoxin-specific IgG and Fab fragments were studied in six baboons. Both IgG and Fab elicited prompt immune responses when injected intramuscularly with Freund's complete adjuvant. Intravenous injection of soluble sheep IgG resulted in a prompt immune response in one baboon while repeated injections caused only a late, weak response in a second animal. Soluble sheep Fab fragments elicited only delayed and weak responses in the two baboons thus challenged. Further immunogenicity studies in ninteen rabbits showed significantly earlier and greater antibody responses to intravenously administered sheep IgG antigen than to Fab fragments derived from the same IgG population. These studies demonstrate that digoxin-specific Fab fragments undergo more rapid and extensive distribution to the extra vascular compartment and also more rapid renal excretion than IgG. Furthermore, Fab fragments are significantly less immunogenic than the parent IgG population. These data indicate potentially important therapeutic advantages for digoxin-specific Fab compared with IgG when administered for the reversal of life-threatening digitlis toxicity.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Silverblatt F. J. Serum disappearance and catabolism of homologous immunoglobulin fragments in rats. Clin Exp Immunol. 1975 Dec;22(3):502–513. [PMC free article] [PubMed] [Google Scholar]
- Butler V. P., Jr, Chen J. P. Digoxin-specific antibodies. Proc Natl Acad Sci U S A. 1967 Jan;57(1):71–78. doi: 10.1073/pnas.57.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler V. P., Jr, Schmidt D. H., Smith T. W., Haber E., Raynor B. D., Demartini P. Effects of sheep digoxin-specific antibodies and their Fab fragments on digoxin pharmacokinetics in dogs. J Clin Invest. 1977 Feb;59(2):345–359. doi: 10.1172/JCI108647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C. An antigen-binding capacity test for human immunoglobulin G (IgG) fragments. J Immunol. 1968 Sep;101(3):433–438. [PubMed] [Google Scholar]
- Curd J., Smith T. W., Jaton J. C., Haber E. The isolation of digoxin-specific antibody and its use in reversing the effects of digoxin. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2401–2406. doi: 10.1073/pnas.68.10.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GITLIN D., KUMATE J., URRUSTI J., MORALES C. THE SELECTIVITY OF THE HUMAN PLACENTA IN THE TRANSFER OF PLASMA PROTEINS FROM MOTHER TO FETUS. J Clin Invest. 1964 Oct;43:1938–1951. doi: 10.1172/JCI105068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold H. K., Smith T. W. Reversal of ouabain and acetyl strophanthidin effects in normal and failing cardiac muscle by specific antibody. J Clin Invest. 1974 Jun;53(6):1655–1661. doi: 10.1172/JCI107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hopkins B. E., Wagner H., Jr, smith T. W. Sodium- and potassium-activated adenosine triphosphatase of the nasal salt gland of the duck (Anas platyrhynchos). Purification, characterization, and NH2-terminal amino acid sequence of the phosphorylating polypeptide. J Biol Chem. 1976 Jul 25;251(14):4365–4371. [PubMed] [Google Scholar]
- Janeway C. A., Merler E., Rosen F. S., Salmon S., Crain J. D. Metabolism of gamma globulin fragments in normal and agammaglobulinemic persons. N Engl J Med. 1968 Apr 25;278(17):919–923. doi: 10.1056/NEJM196804252781701. [DOI] [PubMed] [Google Scholar]
- LATHEM W., DAVIS B. B., ZWEIG P. H., DEW R. The demonstration and localization of renal tubular reabosorption of hemoglobin by stop flow analysis. J Clin Invest. 1960 Jun;39:840–845. doi: 10.1172/JCI104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogielnicki R. P., Waldmann T. A., Strober W. Renal handling of low molecular weight proteins. I. L-Chain metabolism in experimental renal disease. J Clin Invest. 1971 Apr;50(4):901–909. doi: 10.1172/JCI106562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVER J., MACDOWELL M., LEE Y. C. Cellular mechanisms of protein metabolism in the nephron. I. The structural aspects of proteinuria; tubular absorption, droplet formation, and the disposal of proteins. J Exp Med. 1954 Jun 1;99(6):589–604. doi: 10.1084/jem.99.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs H. R., Smith T. W. Reversal of advanced digitoxin toxicity and modification of pharmacokinetics by specific antibodies and Fab fragments. J Clin Invest. 1977 Dec;60(6):1303–1313. doi: 10.1172/JCI108889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLOMON A., WALDMANN T. A., FAHEY J. L., MCFARLANE A. S. METABOLISM OF BENCE JONES PROTEINS. J Clin Invest. 1964 Jan;43:103–117. doi: 10.1172/JCI104884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D. H., Butler V. P., Jr Reversal of digoxin toxicity with specific antibodies. J Clin Invest. 1971 Aug;50(8):1738–1744. doi: 10.1172/JCI106663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz K. M., O'Hara D. S., Smith T. W. Antibody-hapten reaction kinetics: a comparison of hapten interactions with IgG and Fab preparations. J Immunol. 1977 Jun;118(6):1971–1976. [PubMed] [Google Scholar]
- Skubitz K. M., Smith T. W. Determination of antibody-hapten association kinetics: a simplified experimental approach. J Immunol. 1975 Apr;114(4):1369–1374. [PubMed] [Google Scholar]
- Smith T. W., Butler V. P., Jr, Haber E. Characterization of antibodies of high affinity and specificity for the digitalis glycoside digoxin. Biochemistry. 1970 Jan 20;9(2):331–337. doi: 10.1021/bi00804a020. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Haber E., Yeatman L., Butler V. P., Jr Reversal of advanced digoxin intoxication with Fab fragments of digoxin-specific antibodies. N Engl J Med. 1976 Apr 8;294(15):797–800. doi: 10.1056/NEJM197604082941501. [DOI] [PubMed] [Google Scholar]
- Smith T. W. Ouabain-specific antibodies: immunochemical properties and reversal of Na + , K + -activated adenosine triphosphatase inhibition. J Clin Invest. 1972 Jun;51(6):1583–1593. doi: 10.1172/JCI106956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. W., Skubitz K. M. Kinetics in interactions between antibodies and haptens. Biochemistry. 1975 Apr 8;14(7):1496–1502. doi: 10.1021/bi00678a023. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Weigle W. O. The immune response to human gamma-G-immunoglobulin in rabbits unresponsive to Fc fragment and H chain protein. J Immunol. 1967 May;98(5):1020–1027. [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wochner R. D., Strober W., Waldmann T. A. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967 Aug 1;126(2):207–221. doi: 10.1084/jem.126.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmeen D., Ellerson J. R., Dorrington K. J., Painter R. H. The structure and function of immunoglobulin domains. IV. The distribution of some effector functions among the Cgamma2 and Cgamma3 homology regions of human immunoglobulin G1. J Immunol. 1976 Feb;116(2):518–526. [PubMed] [Google Scholar]


