Abstract
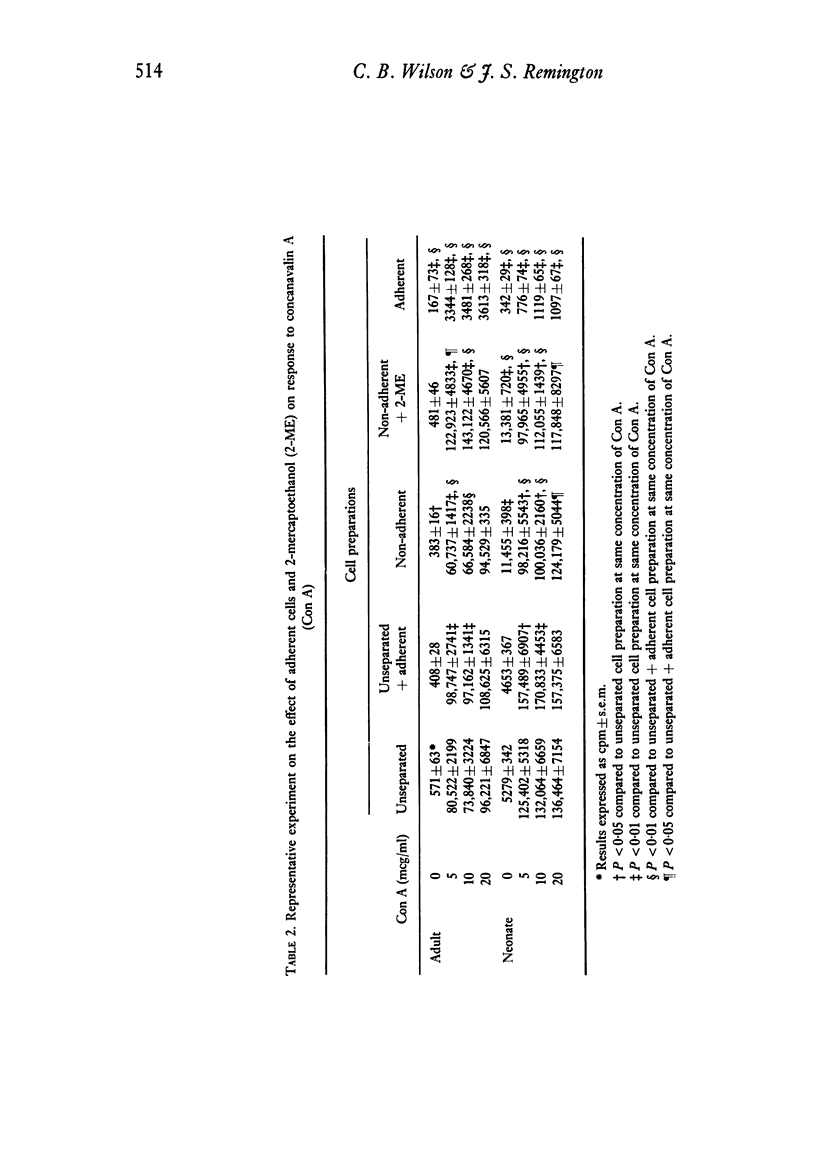
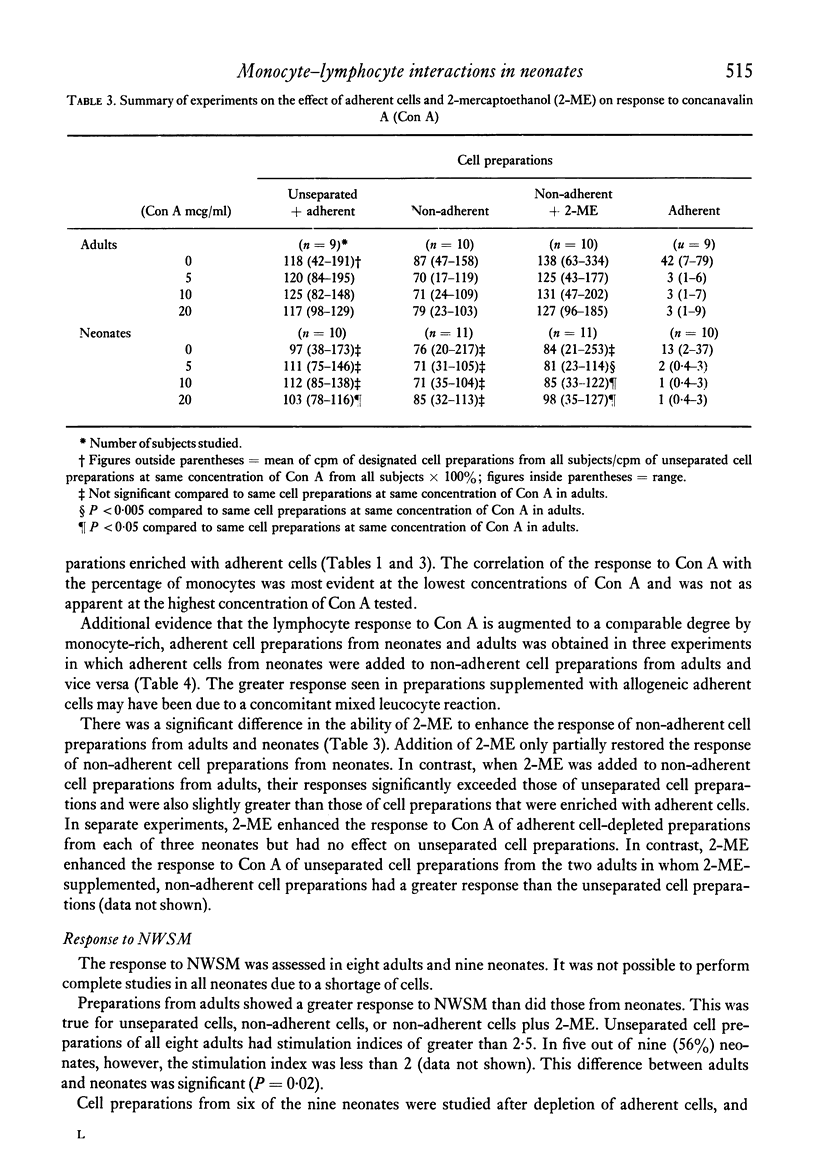
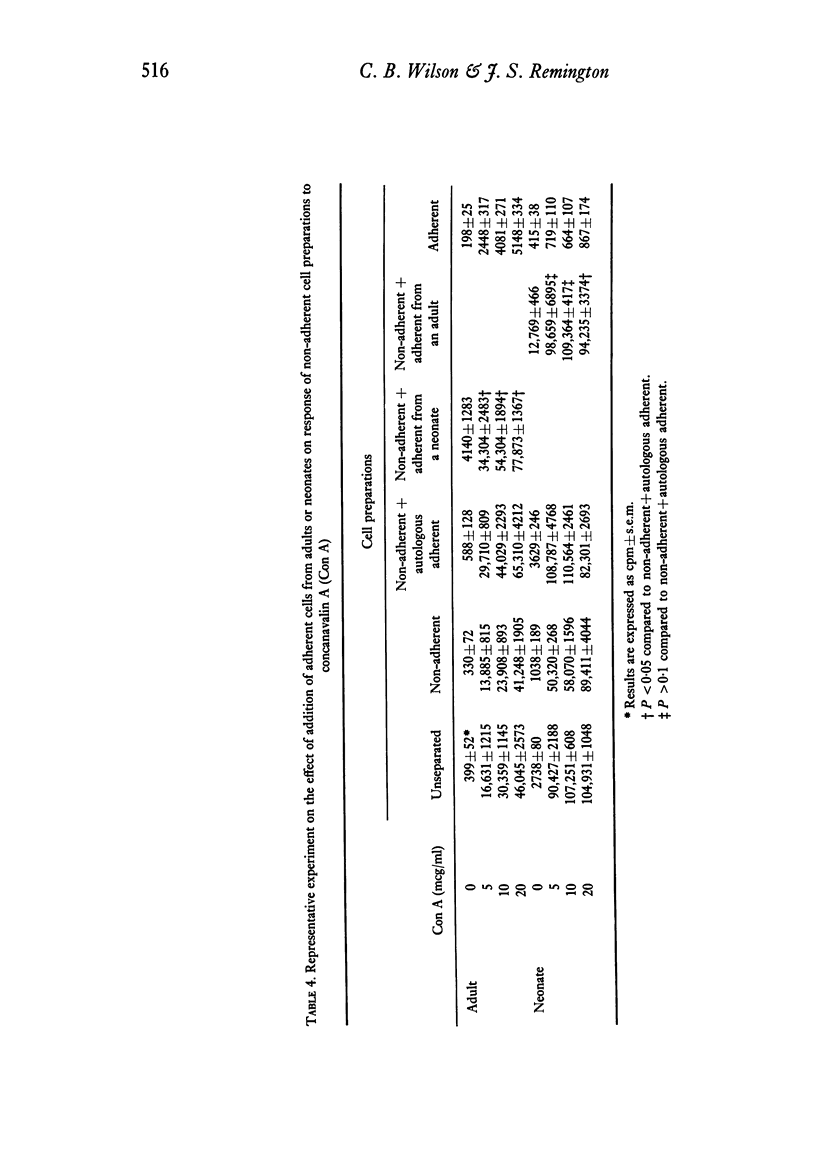
Adherent cells (approximately 75% monocytes, 25% lymphocytes) obtained from neonates and from adults were studied to compare their effects on mitogen-induced lymphocyte transformation. The response of autologous lymphocytes to concanavalin A (Con A) was enhanced significantly by adherent cells from neonates and from adults. Whereas the addition of 2-mercaptoethanol (2-ME) did not enhance the response of unseparated peripheral blood mononuclear cells or fully restore the response of adherent cell-depleted lymphocytes from neonates, both of these effects were observed when 2-ME was added to cell preparations from adults. In fact, the response to Con A of lymphocytes from adults was significantly greater in the presence of 2-ME than in the presence of autologous adherent cells. Equivalent enhancement of the response to Con A was observed when adherent cells from neonates were added to lymphocytes from adults or when adherent cells from adults were added to lymphocytes from neonates.
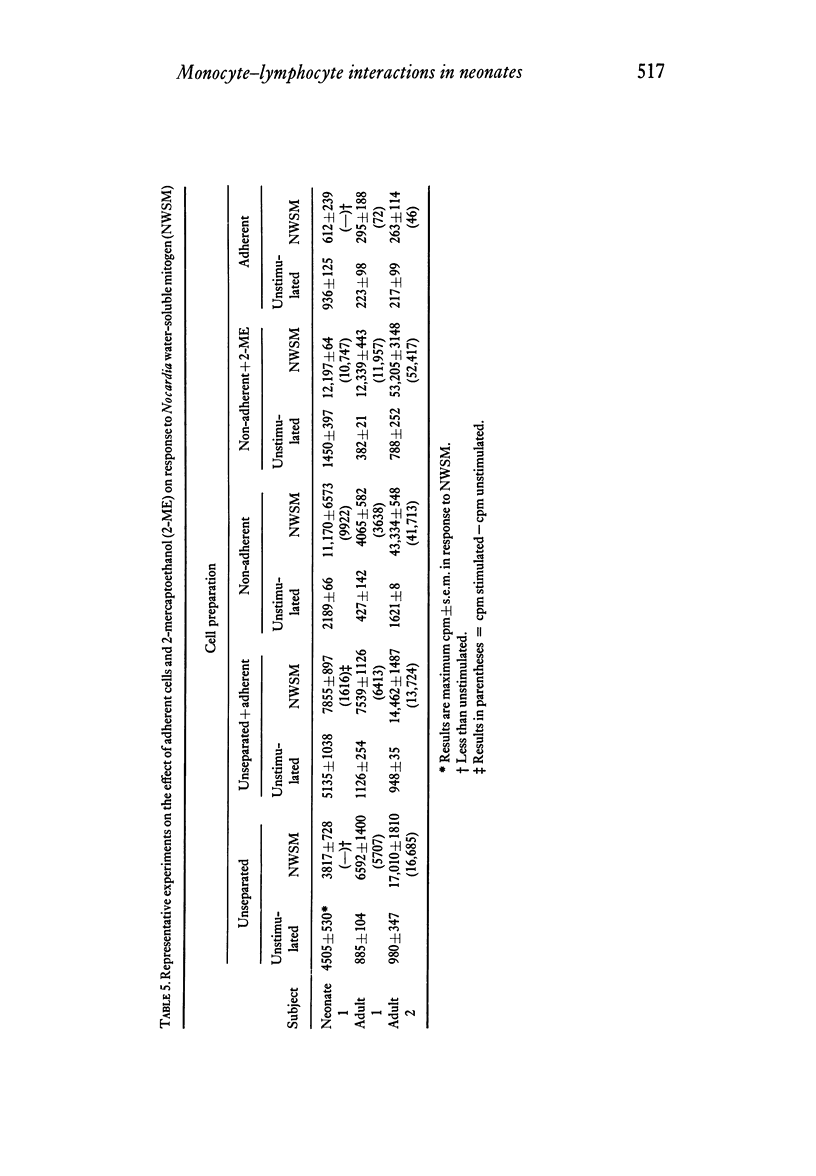
Adherent cells from neonates consistently inhibited the autologous lymphocyte response to the specific B-cell mitogen, NWSM, a water-soluble extract of Nocardia opaca. Lymphocytes from five out of nine neonates failed to respond to NWSM unless adherent cells were depleted. The presence of adherent cells did not prevent the response of lymphocytes from any of the eight adults tested. This difference in response to NWSM between lymphocytes from neonates and adults was significant. Inhibition of the response of autologous lymphocytes to NWSM by adherent cells from adults was of lesser magnitude and could be demonstrated consistently only when 2-ME was added to adherent cell-depleted lymphocyte preparations. We conclude that the effects of adherent cells which were observed were due to monocytes. The enhancing effect of monocytes from adults on lymphocyte response to Con A could be replaced by 2-ME, whereas this was not true for neonates. In contrast to their effects on response to Con A, monocytes from neonates inhibited the response to NWSM more consistently and to a greater degree than did monocytes from adults.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyum A. Separation of lymphocytes, lymphocyte subgroups and monocytes: a review. Lymphology. 1977 Jun;10(2):71–76. [PubMed] [Google Scholar]
- Brochier J., Bona C., Ciorbaru R., Revillard J. P., Chedid L. A human T-independent B lymphocyte mitogen extracted from Nocardia opaca. J Immunol. 1976 Nov;117(5 Pt 1):1434–1439. [PubMed] [Google Scholar]
- Delfraissy J. F., Galanaud P., Dormont J., Wallon C. Primary in vitro antibody response of human peripheral blood lymphocytes: role of phagocytic mononuclear cells. J Immunol. 1978 Apr;120(4):1283–1288. [PubMed] [Google Scholar]
- Fanger M. W., Hart D. A., Wells J. V., Nisonoff A. Enhancement by reducing agents of the transformation of human and rabbit peripheral lymphocytes. J Immunol. 1970 Oct;105(4):1043–1045. [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. II. Cellular interactions in the PFC response of human tonsillar and peripheral blood B lymphocytes to polyclonal activation by pokeweed mitogen. J Immunol. 1976 Dec;117(6):2100–2104. [PubMed] [Google Scholar]
- Forsdyke D. R., David C. M. Comparison of enhancement by heated serum and 2-mercaptoethanol of lymphocyte transformation induced by high concentrations of concanavalin A. Cell Immunol. 1978 Mar 1;36(1):86–96. doi: 10.1016/0008-8749(78)90253-8. [DOI] [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Aging and the immune response. II. Lymphocyte responsiveness and macrophage activation in Toxoplasma gondii-infected mice. J Immunol. 1978 Mar;120(3):944–949. [PubMed] [Google Scholar]
- Hansen G. S., Rubin B., Sorensen S. F. Human leucocyte responses in vitro. I. Transformation of purified T lymphocytes with and without addition of partially purified monocytes. Clin Exp Immunol. 1977 Aug;29(2):295–303. [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Berry D. Functional heterogeneity in macrophages activated by Corynebacterium parvum: characterization of subpopulations with different activities in promoting immune responses and suppressing tumor cell growth. J Immunol. 1977 May;118(5):1530–1540. [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. The induction and regulation of guinea pig B-lymphocyte proliferation in vitro. J Immunol. 1976 Nov;117(5 Pt 1):1594–1602. [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Moretta L. Active thymus derived suppressor lymphocytes in human cord blood. Nature. 1977 Sep 22;269(5626):333–335. doi: 10.1038/269333a0. [DOI] [PubMed] [Google Scholar]
- Potter M. R., Moore M. The effect of adherent and phagocytic cells on human lymphocyte PHA responsiveness. Clin Exp Immunol. 1977 Jan;27(1):159–164. [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Barcinski M. A., Rosenwasser L. J. Function of macrophages in genetic control of immune responsiveness. Fed Proc. 1978 Jan;37(1):79–85. [PubMed] [Google Scholar]
- SHEPARD M. K., WEATHERALL D. J., CONLEY C. L. Semi-quantitative estimation of the distribution of fetal hemoglobin in red cell populations. Bull Johns Hopkins Hosp. 1962 Jun;110:293–310. [PubMed] [Google Scholar]
- Sakane T., Green I. Protein A from Staphylococcus aureus-a mitogen for human T lymphocytes and B lymphocytes but not L lymphocytes. J Immunol. 1978 Jan;120(1):302–311. [PubMed] [Google Scholar]
- Schmidtke J. R., Hatfield S. Activation of purified human thymus-derived (T) cells by mitogens. II. Monocyte- macrophage potentiation of mitogen-induced DNA synthesis. J Immunol. 1976 Feb;116(2):357–362. [PubMed] [Google Scholar]
- Stoecker C. A., Rickard B. M., Abel C. A. Effect of T- and B-lymphocyte mitogens on interactions between lymphocytes and macrophages. Cell Immunol. 1978 Feb;35(2):362–377. doi: 10.1016/0008-8749(78)90156-9. [DOI] [PubMed] [Google Scholar]
- Unsgaard G., Lamvik J. Inhibitory effect of human mononuclear phagocytes on DNA synthesis in stimulated lymphocytes. Acta Pathol Microbiol Scand C. 1977 Oct;85(5):373–380. doi: 10.1111/j.1699-0463.1977.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Cell-mediated immunity and its role in resistance to infection. West J Med. 1977 Jan;126(1):14–31. [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Studies on the regulation of lymphocyte reactivity by normal and activated macrophages. Cell Immunol. 1977 Apr;30(1):108–121. doi: 10.1016/0008-8749(77)90052-1. [DOI] [PubMed] [Google Scholar]
- Wolf R. L., Lominitzer R., Rabson A. R. An inhibitor of lymphocyte proliferation and lymphokine production released by unstimulated foetal monocytes. Clin Exp Immunol. 1977 Mar;27(3):464–468. [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


