Abstract
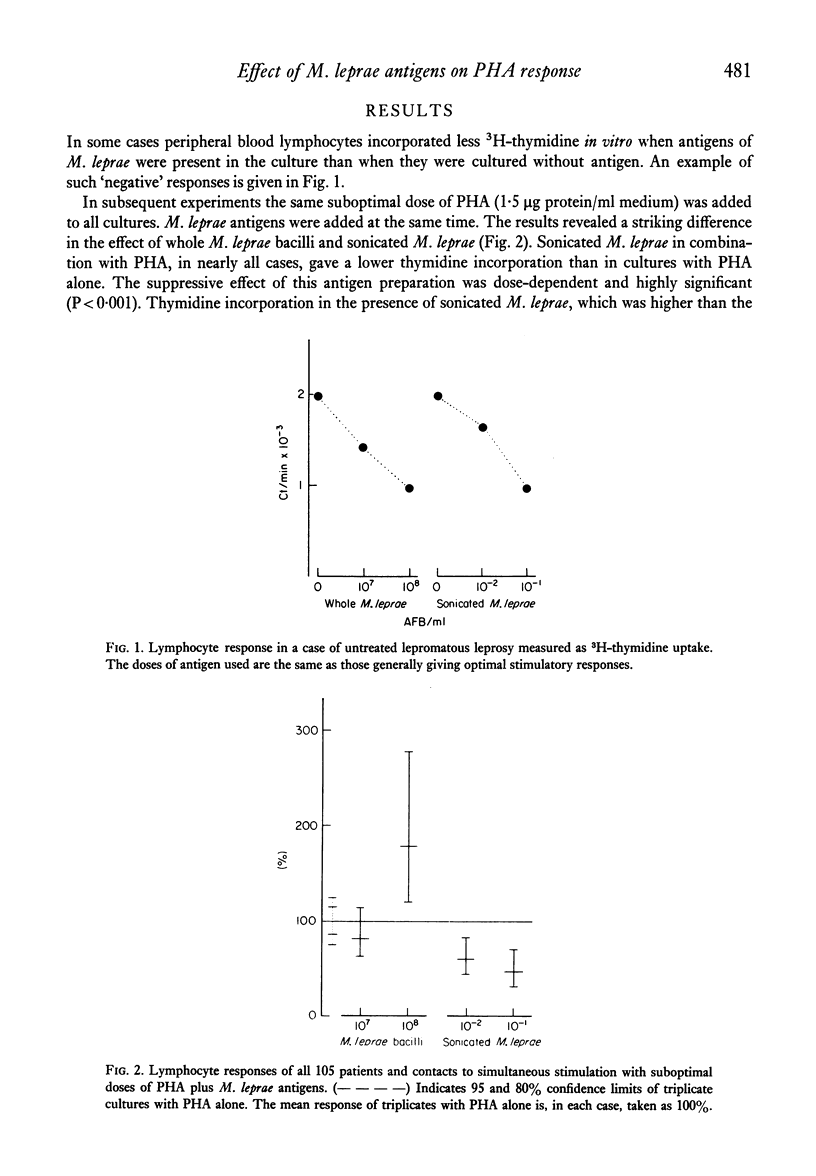
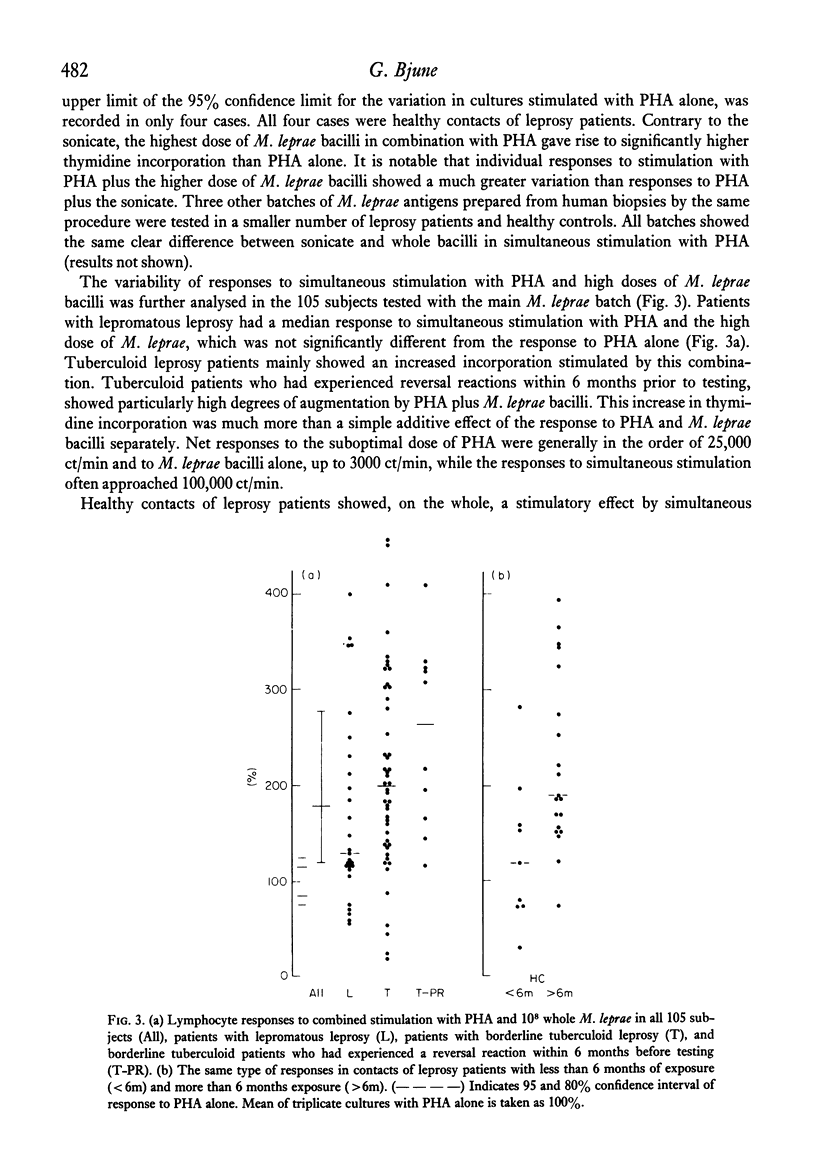
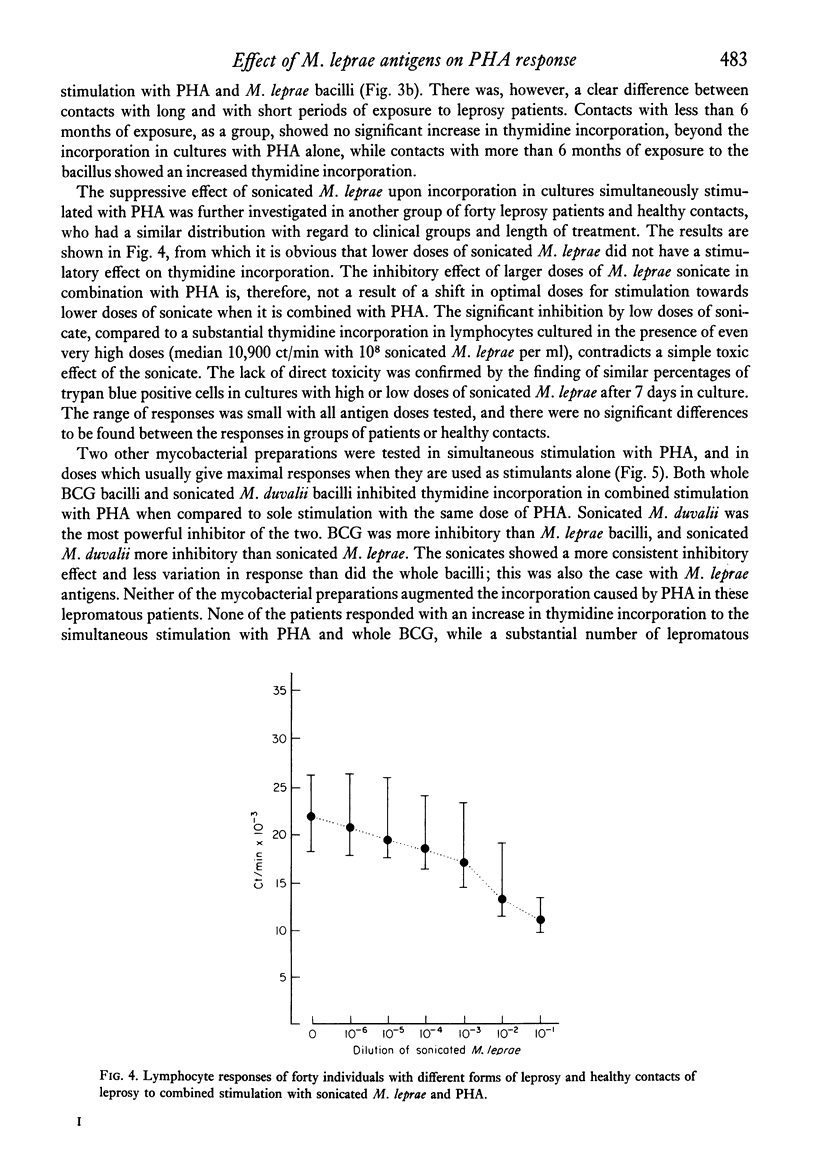
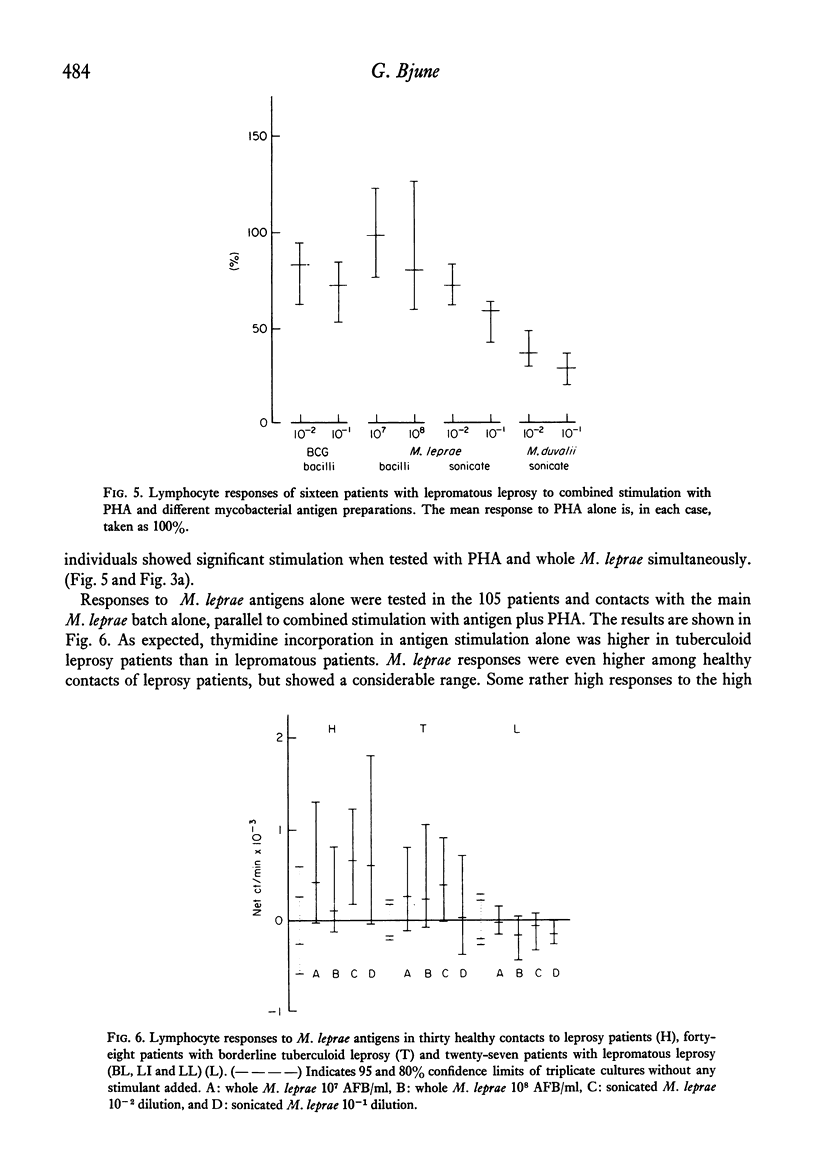
Peripheral blood lymphocytes from 105 subjects with different forms of leprosy and healthy contacts of leprosy patients were stimulated in vitro with different preparations of mycobacterial antigens alone or in combination with a suboptimal dose of phytohaemagglutinin (PHA). In nearly all individuals sonicated leprosy bacilli and PHA together gave a lower 3H-thymidine incorporation than did the same dose of PHA alone. There was no difference in the degree of inhibition seen in the different patient groups or the healthy contacts. High doses of whole, washed Mycobacterium leprae, combined with PHA led to an increased thymidine incorporation in borderline tuberculoid leprosy patients who had experienced a reversal reaction, and in healthy contacts with more than 6 months of exposure, while most lepromatous patients and contacts with less than 6 months exposure did not show an augmentation of the PHA-induced thymidine incorporation. The inhibition exerted by sonicated M. leprae was dose-dependent, seen even with very low doses of antigen, and was not due to direct cytotoxicity. M. bovis, strain BCG, was weakly suppressive in combination with PHA, and sonicated M. duvalii had a very marked suppressive effect. There was no correlation between the suppressive effect of M. leprae antigens and the other mycobacteria neither was there any correlation with the responses to the mycobacterial antigens alone. Many lepromatous leprosy patients showed significant suppression of background incorporation with addition of M. leprae antigens. This paper discusses whether the apparent `non-responsiveness' in lepromatous leprosy could be due to active suppressor mechanisms operative in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen V., Hansen N. E., Karle H., Lind I., Hoiby N., Weeke B. Sequential studies of lymphocyte responsiveness and antibody formation in acute bacterial meningitis. Clin Exp Immunol. 1976 Dec;26(3):469–477. [PMC free article] [PubMed] [Google Scholar]
- Arstila P., Herva E., Ilonen J. Inhibition of in vitro lymphocyte responses by measles virus antigen. Cell Immunol. 1977 Jan;28(1):226–229. doi: 10.1016/s0008-8749(77)80023-3. [DOI] [PubMed] [Google Scholar]
- Azuma I., Taniyama T., Sugimura K., Aladin A., Yamamura Y. Mitogenic activity of the cell walls of mycobacteria, nocardia, corynebacteria and anaerobic coryneforms. Jpn J Microbiol. 1976 Aug;20(4):263–271. doi: 10.1111/j.1348-0421.1976.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Barnetson R. S., Bjune G., Pearson J. M., Kronvall G. Antigenic heterogeneity in patients with reactions in borderline leprosy. Br Med J. 1975 Nov 22;4(5994):435–437. doi: 10.1136/bmj.4.5994.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjune G., Barnetson R. S. Plasma factors in delayed-type hypersensitivity. Augmentation of lymphocyte responses in borderline leprosy reactions. Clin Exp Immunol. 1976 Dec;26(3):397–402. [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Jr, Fasal P. Studies of immune mechanisms in leprosy. 3. The role of cellular and humoral factors in impairment of the in vitro immune response. J Immunol. 1971 Apr;106(4):888–899. [PubMed] [Google Scholar]
- Closs O. In vitro lymphocyte response to purified protein derivative, BCG and Mycobacterium leprae in a population not exposed to leprosy. Infect Immun. 1975 Jun;11(6):1163–1169. doi: 10.1128/iai.11.6.1163-1169.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copperman R., Morton H. E. Reversible inhibition of mitosis in lymphocyte cultures by non-viable Mycoplasma. Proc Soc Exp Biol Med. 1966 Dec;123(3):790–795. doi: 10.3181/00379727-123-31605. [DOI] [PubMed] [Google Scholar]
- Estrada-Parra S. Immunochemical identification of a defined antigen of Mycobacterium leprae. Infect Immun. 1972 Feb;5(2):258–259. doi: 10.1128/iai.5.2.258-259.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah F. S., Lazary S., de Weck A. The effect of Leishmania tropica on stimulation of lymphocytes with phytohaemagglutinin. Immunology. 1976 May;30(5):629–634. [PMC free article] [PubMed] [Google Scholar]
- Godal T., Lofgren M., Negassi K. Immune response to M. leprae of healthy leprosy contacts. Int J Lepr Other Mycobact Dis. 1972 Jul-Sep;40(3):243–250. [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myrvang B., Froland S. S., Shao J., Melaku G. Evidence that the mechanism of immunological tolerance ("central failure") is operative in the lack of host resistance in lepromatous leprosy. Scand J Immunol. 1972;1(4):311–321. doi: 10.1111/j.1365-3083.1972.tb03296.x. [DOI] [PubMed] [Google Scholar]
- Godal T., Myrvang B., Samuel D. R., Ross W. F., Lofgren M. Mechanism of "reactions" in borderline tuberculoid (BT) leprosy. A preliminary report. Acta Pathol Microbiol Scand Suppl. 1973;236(0):45–53. [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjune G., Kronvall G., Axelsen N. H. Mycobacterium leprae specific antibodies detected by radioimmunoassay. Scand J Immunol. 1978;7(2):111–120. doi: 10.1111/j.1365-3083.1978.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Hunt C. V., Khan W., Friedman G., Houck J. C. In vitro inhibition of human peripheral blood lymphocyte transformation by an extract of Pseudomonas putida. Immunology. 1977 Aug;33(2):209–215. [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Miller T. E., Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974 Mar 1;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Lemke H., Flad H. D., Huget R. Inhibition of 3H-thymidine incorporation of lymphocytes by a soluble factor from macrophages. Cell Immunol. 1975 Apr;16(2):379–388. doi: 10.1016/0008-8749(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Roberts D. H. Inhibition of lymphocyte transformation induced by phytohaemagglutinin with porcine mycoplasma. Br Vet J. 1972 Nov;128(11):585–590. doi: 10.1016/s0007-1935(17)36688-5. [DOI] [PubMed] [Google Scholar]
- Rook G. A. The potentiating, mitogenic and inhibitory effects on lymphocytes in vitro, of macrophages in the lymph nodes of mice 'overloaded' with mycobacterial products. Clin Exp Immunol. 1975 Jul;21(1):163–172. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. Three forms of delayed skin-test response evoked by mycobacteria. Nature. 1978 Jan 5;271(5640):64–65. doi: 10.1038/271064a0. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L. Leprosy as a model of subacute and chronic immunologic diseases. J Invest Dermatol. 1976 Sep;67(3):457–463. doi: 10.1111/1523-1747.ep12514734. [DOI] [PubMed] [Google Scholar]
- Uhlenbruck G., Schumacher K., Steinhausen G., Mil A. Tridacnin, a new mitogenic lectin from invertebrate sources. Z Immunitatsforsch Immunobiol. 1977 Sep;153(3):265–267. [PubMed] [Google Scholar]


