Abstract
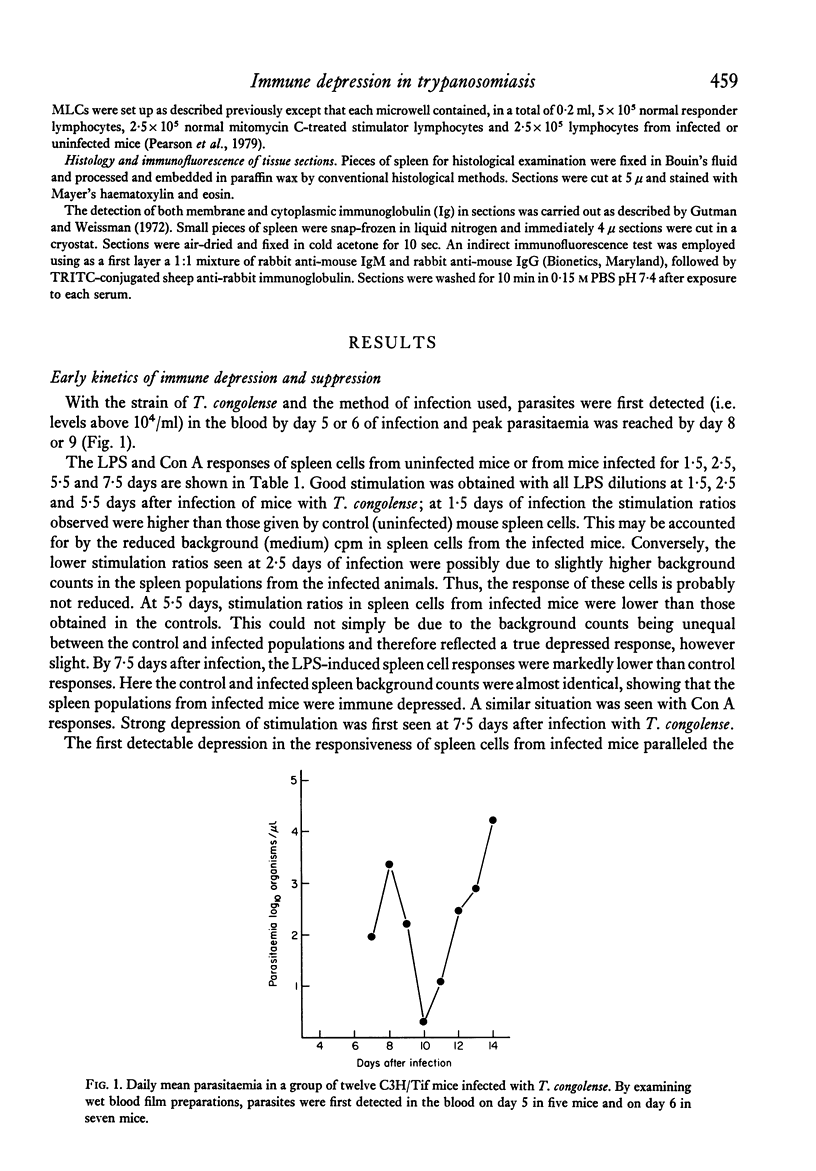
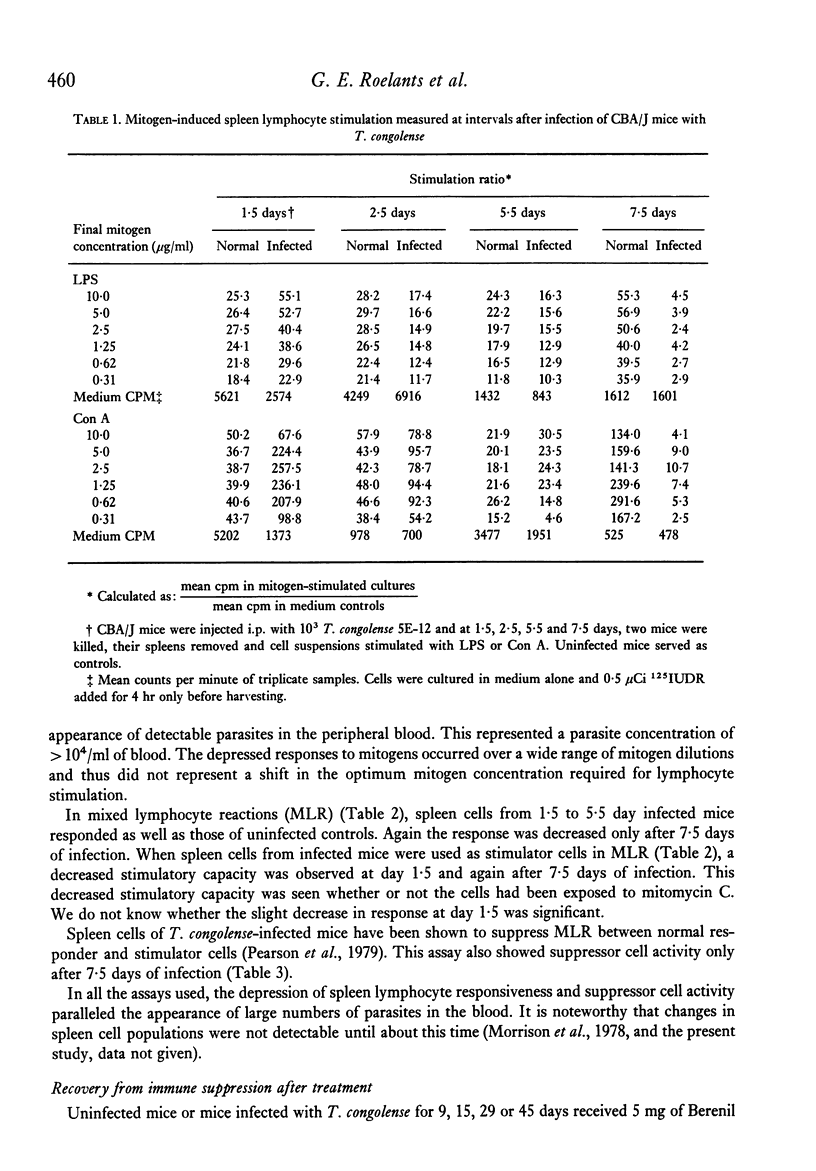
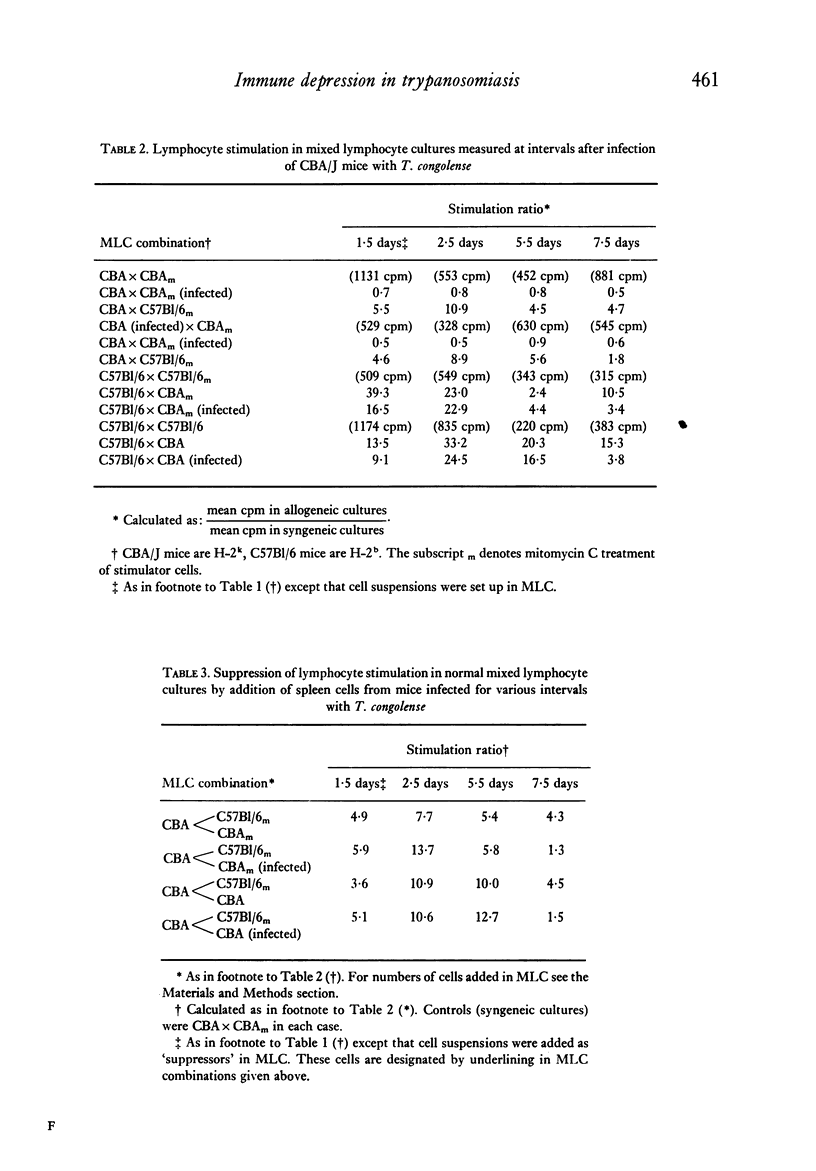
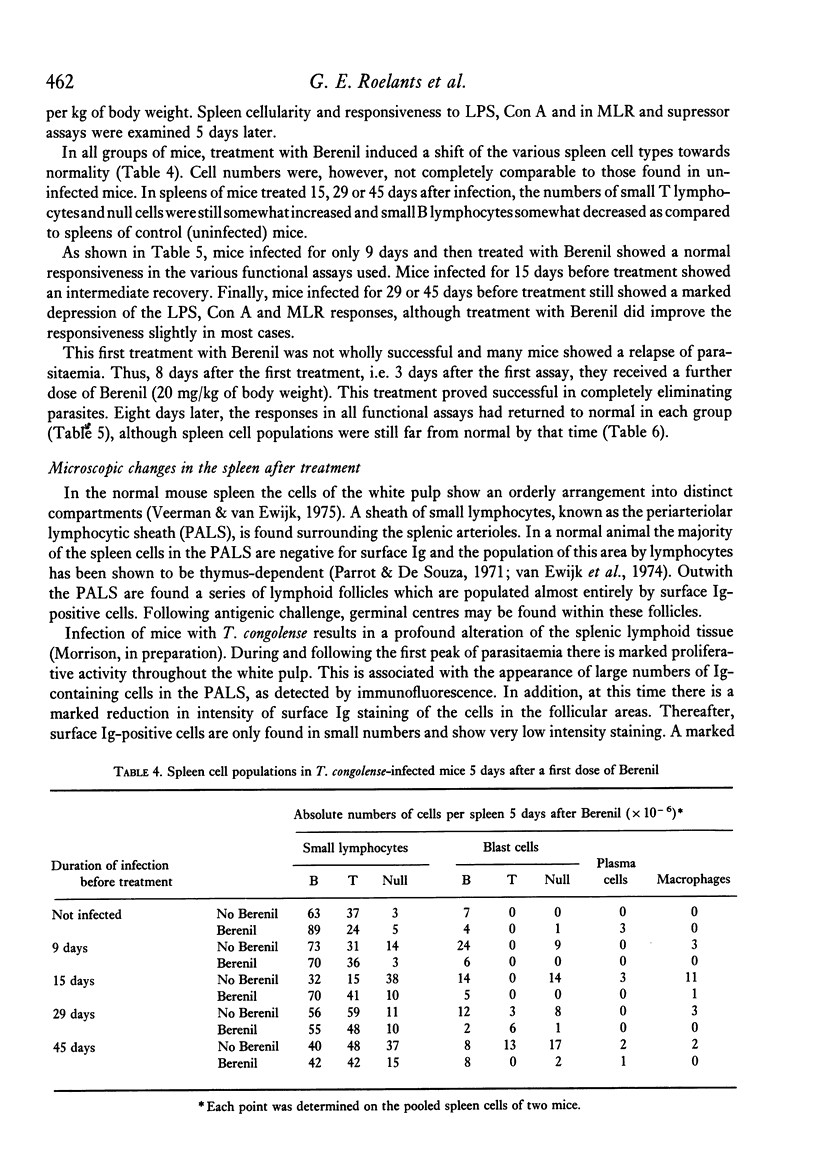
Mice infected with T. congolense were monitored for numbers of parasites in peripheral blood, changes in spleen cell populations, immune depression and suppressor cell activity. Depression of B and T lymphocyte responses and the appearance of suppressor cell activity in spleens of infected mice paralleled the appearance of parasites in the peripheral blood. The immune depression was manifest before any visible changes in spleen cell populations occurred. Treatment of infected mice with the trypanocidal drug Berenil resulted in a rapid clearance of parasites from the peripheral blood, a parallel loss of immune depression and suppressor cell activity and a gradual return towards normal spleen cell composition. The splenic white pulp showed severe depletion following longstanding infection with T. congolense. However, following treatment with Berenil there was rapid repopulation of the white pulp and widespread active germinal centre formation.
Full text
PDF

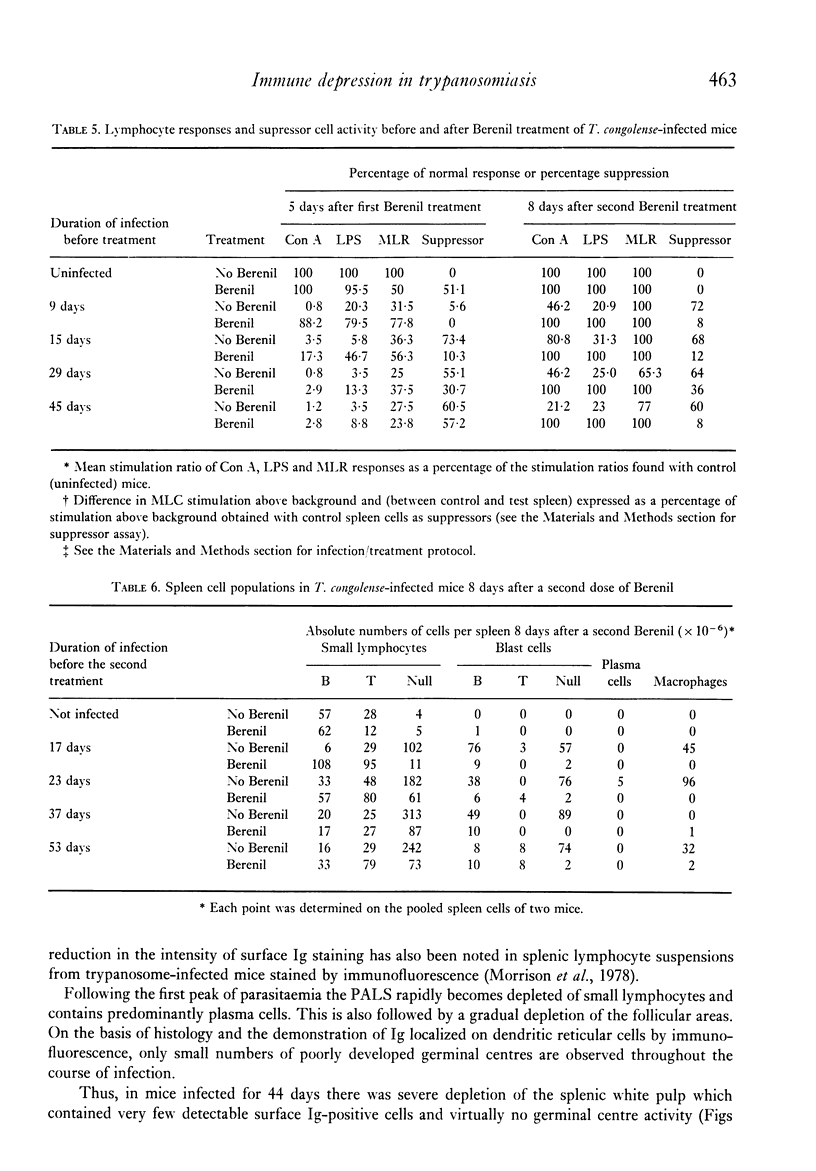

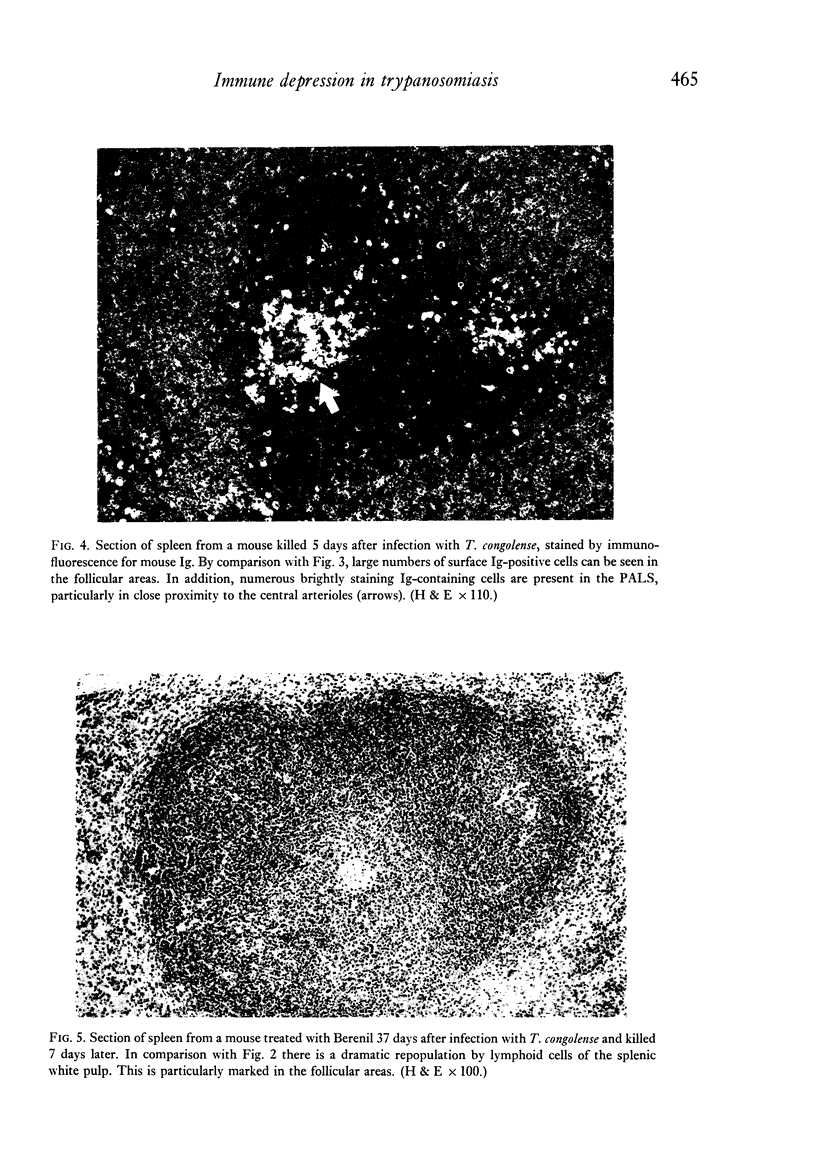




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. F., Albright J. W., Dusanic D. G. Trypanosome-induced splenomegaly and suppression of mouse spleen cell responses to antigen and mitogens. J Reticuloendothel Soc. 1977 Jan;21(1):21–31. [PubMed] [Google Scholar]
- Albright J. W., Albright J. F., Dusanic D. G. Mechanisms of trypanosome-mediated suppression of humoral immunity in mice. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3923–3927. doi: 10.1073/pnas.75.8.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askonas B. A., Corsini A. C., Clayton C. E., Ogilvie B. M. Functional depletion of T- and B-memory cells and other lymphoid cell subpopulations-during trypanosomiasis. Immunology. 1979 Feb;36(2):313–321. [PMC free article] [PubMed] [Google Scholar]
- Chernyakhovskaya I. Y., Shaghijan H. S., Slavina E. G., Svet-Moldavsky G. J. Helminths and allotransplantation. Rev Eur Etud Clin Biol. 1972 Apr;17(4):395–399. [PubMed] [Google Scholar]
- Colley D. G., Hieny S. E., Bartholomew R. K., Cook J. A. Immune responses during human schistosomiasis mansoni. III. Regulatory effect of patient sera on human lymphocyte blastogenic responses to schistosome antigen preparations. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):917–925. [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Goodgame R. W. Immune responses during human schistosomiasis mansoni. IV. Induction of suppressor cell activity by schistosome antigen preparations and concanavalin A. J Immunol. 1978 Apr;120(4):1225–1232. [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Dessaint J. P., Camus D., Fischer E., Capron A. Inhibition of lymphocyte proliferation by factor(s) produced by Schistosoma mansoni. Eur J Immunol. 1977 Sep;7(9):624–629. doi: 10.1002/eji.1830070909. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Jayawardena A. N. Suppressor cells in mice infected with Trypanosoma brucei. J Immunol. 1977 Sep;119(3):1029–1033. [PubMed] [Google Scholar]
- Faubert G., Tanner C. E. Trichinella spiralis: inhibition of sheep hemagglutinins in mice. Exp Parasitol. 1971 Aug;30(1):120–123. doi: 10.1016/0014-4894(71)90077-4. [DOI] [PubMed] [Google Scholar]
- Golenser J., Spira D. T., Zuckerman A. Dynamics of thymidine incorporation by spleen cells from rats infected with Plasmodium berghei. Clin Exp Immunol. 1975 Nov;22(2):364–371. [PMC free article] [PubMed] [Google Scholar]
- Goodwin L. G., Green D. G., Guy M. W., Voller A. Immunosuppression during trypanosomiasis. Br J Exp Pathol. 1972 Feb;53(1):40–43. [PMC free article] [PubMed] [Google Scholar]
- Goodwin L. G. The pathology of African trypanosomiasis. Trans R Soc Trop Med Hyg. 1970;64(6):797–817. doi: 10.1016/0035-9203(70)90096-9. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Whittle H. C., Molyneux D. H. Immunosuppression in Gambian trypanosomiasis. Trans R Soc Trop Med Hyg. 1973;67(6):846–850. doi: 10.1016/0035-9203(73)90013-8. [DOI] [PubMed] [Google Scholar]
- Gutman G. A., Weissman I. L. Lymphoid tissue architecture. Experimental analysis of the origin and distribution of T-cells and B-cells. Immunology. 1972 Oct;23(4):465–479. [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H., Eardley D. D. Activation of distinct helper and suppressor T cells in experimental trypanosomiasis. J Immunol. 1978 Aug;121(2):622–628. [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Humphrey J. H. The generation of memory cells. I. The role of C3 in the generation of B memory cells. Immunology. 1977 Jul;33(1):31–40. [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G. The generation of memory cells. II. Generation of B memory cells with preformed antigen-antibody complexes. Immunology. 1978 Apr;34(4):643–652. [PMC free article] [PubMed] [Google Scholar]
- Longstaffe J. A., Freeman J., Hudson K. M. Immunosuppression in trypanosomiasis: some thymus dependent and thymus independent responses. Trans R Soc Trop Med Hyg. 1973;67(2):264–265. doi: 10.1016/0035-9203(73)90169-7. [DOI] [PubMed] [Google Scholar]
- Loor F., Roelants G. E. Immunofluorescence studies of a possible prethymic T-cell differentiation in congenitally athymic (nude) mice. Ann N Y Acad Sci. 1975 Jun 30;254:226–241. doi: 10.1111/j.1749-6632.1975.tb29173.x. [DOI] [PubMed] [Google Scholar]
- Losos G. J., Paris J., Wilson A. J., Dar F. K. Distribution of Trypanosoma congolense in tissues of cattle. Trans R Soc Trop Med Hyg. 1973;67(2):278–278. doi: 10.1016/0035-9203(73)90193-4. [DOI] [PubMed] [Google Scholar]
- Mansfield J. M., Wallace J. H. Suppression of cell-mediated immunity in experimental African trypanosomiasis. Infect Immun. 1974 Aug;10(2):335–339. doi: 10.1128/iai.10.2.335-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison W. I., Roelants G. E., Mayor-Withey K. S., Murray M. Susceptibility of inbred strains of mice to Trypanosoma congolense: correlation with changes in spleen lymphocyte populations. Clin Exp Immunol. 1978 Apr;32(1):25–40. [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. I. The role of the macrophage. Immunology. 1974 Nov;27(5):815–824. [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- Parrott D. M., De Sousa M. Thymus-dependent and thymus-independent populations: origin, migratory patterns and lifespan. Clin Exp Immunol. 1971 May;8(5):663–684. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. W., Roelants G. E., Pinder M., Lundin L. B., Mayor-Withey K. S. Immune depression in trypanosome-infected mice. III. suppressor cells. Eur J Immunol. 1979 Mar;9(3):200–204. doi: 10.1002/eji.1830090306. [DOI] [PubMed] [Google Scholar]
- Pelley R. P., Ruffier J. J., Warren K. S. Suppressive effect of a chronic helminth infection, schistosomiasis mansoni, on the in vitro responses of spleen and lymph node cells to the T cell mitogens phytohemagglutinin and concanavalin A. Infect Immun. 1976 Apr;13(4):1176–1183. doi: 10.1128/iai.13.4.1176-1183.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants G. E., Loor F., von Boehmer H., Sprent J., Hägg L. B., Mayor K. S., Rydén A. Five types of lymphocytes (Ig-theta-, Ig-theta+weak, Ig-theta+strong, Ig+theta- and Ig+theta+) characterized by double immunofluorescence and electrophoretic mobility. Organ distribution in normal and nude mice. Eur J Immunol. 1975 Feb;5(2):127–131. doi: 10.1002/eji.1830050211. [DOI] [PubMed] [Google Scholar]
- Roelants G. E., Pearson T. W., Tyrer H. W., Mayor-Withey K. S., Lundin L. B. Immune depression in trypanosome-infected mice. II. Characterization of the spleen cell types involved. Eur J Immunol. 1979 Mar;9(3):195–199. doi: 10.1002/eji.1830090305. [DOI] [PubMed] [Google Scholar]
- Salaman M. H., Wedderburn N., Bruce-Chwatt L. J. The immunodepressive effect of a murine plasmodium and its interaction with murine oncogenic viruses. J Gen Microbiol. 1969 Dec;59(3):383–391. doi: 10.1099/00221287-59-3-383. [DOI] [PubMed] [Google Scholar]
- Shimp R. G., Crandall R. B., Crandall C. A. Heligmosomoides polygyrus (=Nematospiroides dubius): suppression of antibody response to orally administered sheep erythrocytes in infected mice. Exp Parasitol. 1975 Oct;38(2):257–269. doi: 10.1016/0014-4894(75)90028-4. [DOI] [PubMed] [Google Scholar]
- Veerman A. J., van Ewijk W. White pulp compartments in the spleen of rats and mice. A light and electron microscopic study of lymphoid and non-lymphoid celltypes in T- and B-areas. Cell Tissue Res. 1975;156(4):417–441. doi: 10.1007/BF00225103. [DOI] [PubMed] [Google Scholar]
- Weller P. F. Cell-mediated immunity in experimental filariasis: lymphocyte reactivity to filarial stage-specific antigens and to B- and T-cell mitogens during acute and chronic infection. Cell Immunol. 1978 May;37(2):369–382. doi: 10.1016/0008-8749(78)90205-8. [DOI] [PubMed] [Google Scholar]




