Abstract
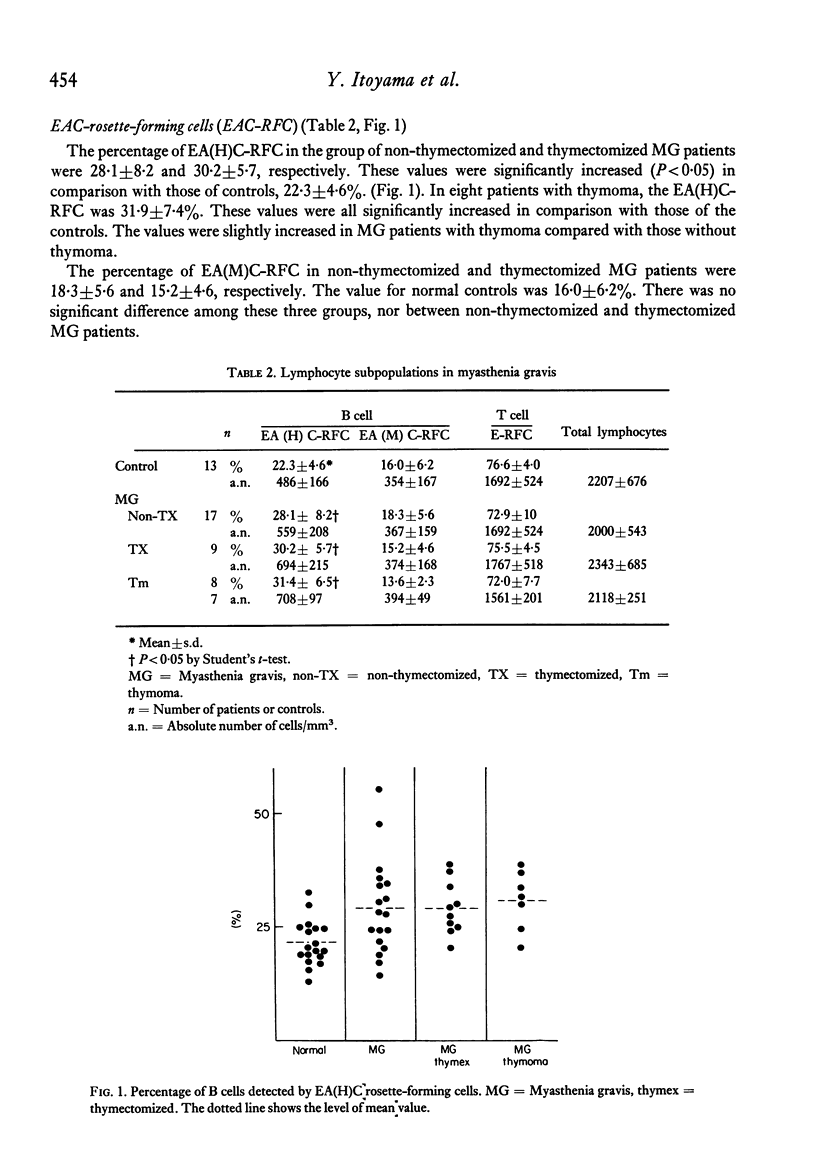
Peripheral blood lymphocytes from seventeen non-thymectomized and nine thymectomized patients with myasthenia gravis (MG) and thirteen healthy controls were examined for the presence of surface markers characteristic of T and B lymphocytes by rosette formation with sheep red blood cells (SRBC). T cells were identified by their capacity to spontaneously form rosettes with SRBCs. The percentage of B lymphocytes was determined by the erythrocyte antibody complement (EAC) rosette-forming test. The EAC complex was prepared with either whole rabbit anti-SRBC serum or with the IgM fraction of rabbit anti-SRBC serum. The two kind of erythrocyte complement rosette-forming cells (EAC-RFC) are designated erythrocyte-haemolysin-complement RFC (EA(H)C-RFC), and erythrocyte-IgM-complement RFC (EA(M)C-RFC). The percentage of total lymphocytes and T cells was not altered in MG patients. The percentage of 'active' T cells, which have been considered to be more actively involved in cellular immunity, was also similar in MG patients and controls. A significant increase in EA(H)C-RFC occurred in both thymectomized and non-thymectomized MG patients, while in B cells detected by EA(M)C-RFC no alterations were found. The increase in EA(H)C-RFC in lymphocytes from MG patients may be due to an increase in the 19S antibody-forming B lymphocytes or to an increase in T cells which have Fc receptors on their surface.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Lisak R. P., Zweiman B., Abrahamsohn I., Penn A. S. The thymus in myasthenia gravis. Evidence for altered cell populations. N Engl J Med. 1974 Dec 12;291(24):1271–1275. doi: 10.1056/NEJM197412122912403. [DOI] [PubMed] [Google Scholar]
- Abramsky O., Aharonov A., Webb C., Fuchs S. Cellular immune response to acetylcholine receptor-rich fraction, in patients with myasthenia gravis. Clin Exp Immunol. 1975 Jan;19(1):11–16. [PMC free article] [PubMed] [Google Scholar]
- Alpert L. I., Rule A., Norio M., Kott E., Kornfeld P., Osserman K. E. Studies in myasthenia gravis: cellular hypersensitivity to skeletal muscle. Am J Clin Pathol. 1972 Dec;58(6):647–653. doi: 10.1093/ajcp/58.6.647. [DOI] [PubMed] [Google Scholar]
- Bender A. N., Ringel S. P., Engel W. K., Daniels M. P., Vogel Z. Myasthenia gravis: a serum factor blocking acetylcholine receptors of the human neuromuscular junction. Lancet. 1975 Mar 15;1(7907):607–609. doi: 10.1016/s0140-6736(75)91886-3. [DOI] [PubMed] [Google Scholar]
- Gergely P., Bakács T., Cornain S., Klein E. Fc receptors on human blood B lymphocytes. Clin Exp Immunol. 1977 Apr;28(1):99–102. [PMC free article] [PubMed] [Google Scholar]
- Goust J. M., Castaigne A., Moulias R. Delayed hypersensitivity to muscle and thymus in myasthenia gravis and polymyositis. Clin Exp Immunol. 1974 Sep;18(1):39–47. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Wigzell H., Aiuti F. Human lymphocyte subpopulations: classification according to surface markers and-or functional characteristics. Transplant Rev. 1973;16:163–195. doi: 10.1111/j.1600-065x.1973.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Kawanami S., Itoyama Y., Kuroiwa Y. Leucocyte migration inhibition in myasthenia gravis. The effect of thymectomy. J Neurol. 1976 Jul 15;213(1):33–40. doi: 10.1007/BF00316337. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Fulpius B. W. A radioimmunoassay for the quantitative evaluation of anti-human acetylcholine receptor antibodies in myasthenia gravis. Clin Exp Immunol. 1977 Jul;29(1):16–22. [PMC free article] [PubMed] [Google Scholar]
- NASTUK W. L., PLESCIA O. J., OSSERMAN K. E. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960 Oct;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- NASTUK W. L., PLESCIA O. J., OSSERMAN K. E. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960 Oct;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- Papatestas A. E., Alpert L. I., Osserman K. E., Osserman R. S., Kark A. E. Studies in myasthenia gravis: effects of thymectomy. Results on 185 patients with nonthymomatous and thymomatous myasthenia gravis, 1941-1969. Am J Med. 1971 Apr;50(4):465–474. doi: 10.1016/0002-9343(71)90336-6. [DOI] [PubMed] [Google Scholar]
- Parish C. R. Separation and functional analysis of subpopulations of lymphocytes bearing complement and Fc receptors. Transplant Rev. 1975;25:98–120. doi: 10.1111/j.1600-065x.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- RUSSELL D. S. Histological changes in the striped muscles in myasthenia gravis. J Pathol Bacteriol. 1953 Apr;65(2):279–289. doi: 10.1002/path.1700650202. [DOI] [PubMed] [Google Scholar]
- Simpson J. A. Myasthenia gravis as an autoimmune disease: clinical aspects. Ann N Y Acad Sci. 1966 Jan 26;135(1):506–516. doi: 10.1111/j.1749-6632.1966.tb45499.x. [DOI] [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yata J., Tsukimoto I., Tachibana T. Human lymphocyte subpopulations. Human thymus-lymphoid tissue (HTL) antigen-positive lymphocytes forming rosettes with sheep erythrocytes and HTL antigen-negative lymphocytes interacting with antigen-antibody-complement complexes. Clin Exp Immunol. 1973 Jul;14(3):319–326. [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]


