Abstract
The ability of unsaturated fatty acid methyl esters to modify amino acid residues in bovine serum albumin (BSA), glutamine synthetase, and insulin in the presence of a metal-catalyzed oxidation system [ascorbate/Fe(III)/O2] depends on the degree of unsaturation of the fatty acid. The fatty acid-dependent generation of carbonyl groups and loss of lysine residues increased in the order methyl linoleate < methyl linolenate < methyl arachidonate. The amounts of alkyl hydroperoxides, malondialdehyde, and a number of other aldehydes that accumulated when polyunsaturated fatty acids were oxidized in the presence of BSA were significantly lower than that observed in the absence of BSA. Direct treatment of proteins with various lipid hydroperoxides led to a slight increase in the formation of protein carbonyl derivatives, whereas treatment with the hydroperoxides together with Fe(II) led to a substantial increase in the formation of protein carbonyls. These results are consistent with the proposition that metal-catalyzed oxidation of polyunsaturated fatty acids can contribute to the generation of protein carbonyls by direct interaction of lipid oxidation products (α,β-unsaturated aldehydes) with lysine residues (Michael addition reactions) and also by interactions with alkoxyl radicals obtained by Fe(II) cleavage of lipid hydroperoxides that are formed. In addition, saturated aldehydes derived from the polyunsaturated fatty acids likely react with lysine residues to form Schiff base adducts.
It is well established that end-products of lipid peroxidation, such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal, cause protein damage by means of reactions with lysine amino groups, cysteine sulfhydryl groups, and histidine imidazole groups (1–5). Modifications of biomolecules by aldehyde products of lipid peroxidation are also believed to contribute to lipofuscin formation, as seen in aging and in the progression of some degenerative diseases. A fluorescent product, a 2-hydroxy-3-imino-1,2-dihydropyrrol derivative, is formed upon oxidative cyclization of the 2:1 lysine:4-hydroxy-2-nonenal Michael adduct–Schiff base cross-link (6). Recent studies have shown that 4-hydroxy-2-nonenal is involved in stress signaling pathways by inhibiting the NF-κB/Rel system (7) and by activation of c-Jun N-terminal kinases (8).
The reaction of the primary lipid peroxidation products, lipid hydroperoxides (LOOH), with proteins is not well characterized. This is presumably because of low stability of LOOH, which decomposes rapidly via radical intermediates to aldehydic products (9, 10). It is suggested that the reaction of LOOH with proteins does not involve alterations of the lipid moiety (11), but no chemical characterization of such products has been carried out. An antibody raised against lipid peroxide-modified protein was shown to cross-react with proteins and cells after treatment with LOOH and with proteins in human atherosclerotic lesions, but it did not cross-react with aldehyde-modified proteins (12). Lipid hydroperoxides were also found to inhibit plasma lecithin:cholesterol acyltransferase (13). Recently, the ability of linoleic-13-hydroperoxide to convert BSA to carbonyl derivatives in the presence of either Cu2+ or Fe3+ has been demonstrated in this laboratory (14).
Here, we demonstrate that the fatty acid-dependent protein modifications, generation of carbonyl derivatives, and loss of lysine residues are dependent on the degree of unsaturation of the fatty acid and that polyunsaturated fatty acid alkoxyl radicals formed during degradation of lipid hydroperoxides are likely involved in the formation of protein carbonyl derivatives.
Materials and Methods
Materials.
Bovine serum albumin (BSA), bovine insulin, and soybean lipoxygenase (type I-b) were from Sigma. Glutamine synthetase (GS) was from Escherichia coli YMC 10 pgln6, which overproduces the enzyme. The enzyme was purified by the zinc aggregation procedure as described (15). Methyl esters of stearic acid, oleic acid, linoleic acid, linolenic acid, and arachidonic acid were from Sigma. 15(S)-hydroperoxy-(E,Z,Z,Z)-5,8,11,13-eicosatetraenoic acid (15HpETE) was obtained from Cayman Chemical.
Preparation of Lipid Hydroperoxides.
The 13(S)-hydroperoxides were prepared by enzymatic reaction of lipoxygenase with methyl linoleate or methyl linolenate (16). Mixtures of lipid hydroperoxides were prepared by incubation of 10 mM methyl linoleate or 10 mM methyl linolenate with 25 mM ascorbate and 0.1 mM FeCl3 in 50 mM Hepes buffer, pH 7.2, at 37°C for 2 hr. The lipid hydroperoxides were purified according to Reeder and Wilson (16). The lipid hydroperoxide fractions were detected by absorbance at 234 nm and the purity was determined by GC–MS. Based on total ion counts, the 13(S)-hydroperoxides were 99% pure. The lipid hydroperoxides were stored in ethanol at −20°C.
Incubation with Methyl Esters.
Protein (1–2 mg/ml) was incubated with stirring at 37°C in 50 mM phosphate or 50 mM Hepes buffer, pH 7.2. Reactive oxygen species were generated by addition of 25 mM ascorbate and 0.1 mM FeCl3 (17). Fatty acid methyl esters were added at final concentrations of 0.04 mM to 100 mM. PD10 columns (Pharmacia) were used to remove ascorbate, free iron, and the fatty acids. The removal of the fatty acids was confirmed by determination of the lipid content before and after the PD10 column by gravimetry. Lipid was removed before analyses of protein carbonyl and amino acid composition.
Incubation with Lipid Hydroperoxides.
Protein (2 mg/ml) was incubated with stirring at 37°C in 50 mM Hepes buffer, pH 7.2, with 10 mM lipid hydroperoxide [ɛ234 = 2.5 × 10 M−1⋅cm−1 (16)]. The concentration of ferrous ions was 1 mM. PD10 columns were used to remove lipid hydroperoxide and iron after incubation.
Incubation with Aldehydes.
Protein (2 mg/ml) was incubated with stirring at 37°C in 50 mM Hepes buffer, pH 7.2, with 2 μM or 2 mM aldehyde (Sigma). The aldehydes were removed after incubation by protein precipitation with 10% trichloroacetic acid (TCA).
Reduction of Protein-Lipid Derivatives.
For reduction of Schiff base derivatives, the protein-lipid adducts (5 mg/ml protein) were incubated with 25 mM NaCNBH3 in 0.25 M phosphate buffer, pH 6.0, for 1 hr at 37°C (18). For reduction of protein carbonyl, 16 mg/ml protein was incubated with 50 mM NaBH4 in 0.1 mM Tris⋅HCl containing 1 mM EDTA for 1 hr at 37°C (19).
Protein Carbonyl.
The protein carbonyl content was determined by the reaction with 2,4-dinitrophenylhydrazine (DNP) as described by Levine et al. (20). One milligram of protein was incubated for 10 min with 10 mM DNP in 2 M HCl. For each sample, a blank without DNP was run in parallel. After precipitation with trichloroacetic acid, the pellet was washed three times with ethanol/ethyl acetate (1:1, vol/vol). The carbonyl content was calculated from the absorbance of the protein-2,4-dinitrophenylhydrazone derivative at 370 nm.
Amino Acid Analysis.
Protein (10 μg) was hydrolyzed at 155°C for 45 min after addition of 200 μl of 6 M constant-boiling HCl (21). Amino acid composition of the acid hydrolysate was determined by precolumn o-phthaldialdehyde derivatization and HPLC with fluorescence detection (22).
Analysis of Nɛ-(Carboxymethyl)lysine (CML).
Ascorbate, iron, and lipid were separated from protein by use of PD10 columns, and solutions were dried by centrifugal evaporation. Protein (3 mg) was hydrolyzed as described above and the amino acids were converted to their N,O-trifluoroacetyl methyl ester derivatives for GC–MS as described by Knecht et al. (23). The GC–MS analyses were performed by using a gas chromatograph equipped with a 30-m HP-5MS capillary column coupled to a HP 5971A mass selective detector (Hewlett Packard). The temperature program was 2 min at 150°C, then 5°C/min to 180°C and 15°C/min to 250°C, and holding time was 5 min. A CML standard was kindly provided by J. R. Requena (Laboratory of Biochemistry, National Heart, Lung, and Blood Institute).
Lipid Hydroperoxides.
Aliquots of incubation mixtures were extracted with heptane. The lipid hydroperoxide concentration in the heptane phase was determined by use of a colorimetric method with KI and starch (24). H2O2 was used as standard.
Aldehydic Lipid Peroxidation Products.
Volatile aldehydes were collected from the incubation mixtures by dynamic headspace sampling. Because BSA promoted the release of volatiles, 2 mg/ml BSA was added to samples without protein just before the headspace sampling. The samples were purged with nitrogen at 340 ml/min for 10 min at 45°C and the volatiles were trapped on Tenax-GR traps (Chrompack). The volatiles were released from the Tenax traps by thermal desorption (ATD-400, Perkin–Elmer) and analyzed by GC–MS on a 30-m DB 1701 capillary column (J & W Scientific, Folsom, CA). The following temperature program was used: 65°C for 1 min, ramping 4°C/min to 90°C and 20°C/min to 240°C, and holding 5 min at the final temperature. The ionization energy of the mass spectrometer was set at 70 eV in EI mode and the detector operated with a mass range of 30–250 atomic mass units with repetition rate at 3.4 scans per s. For quantification purposes, aldehydes dissolved in ethanol were added in five sets of concentrations to Hepes buffer, pH 7.2. The standards were collected and analyzed as described above.
MDA.
MDA was measured by use of a GC method after derivatization of MDA with pentafluorophenylhydrazine as described by Yeo et al. (25). A gas chromatograph equipped with a 30-m HP-5MS capillary column coupled to a flame ionization detector was used.
Results
Protein Modification.
Protein carbonyl formation.
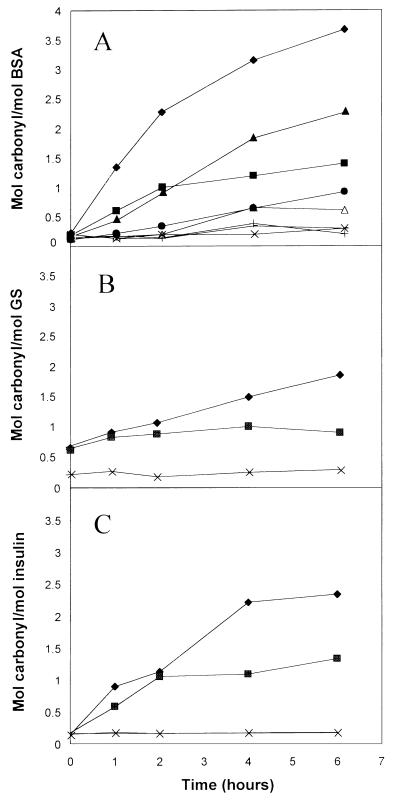
To study the effects of polyunsaturated fatty acids on the oxidative modification of proteins, we measured the carbonyl content of proteins after their exposure to the ascorbate and Fe3+ mixed-function oxidation systems, in both the presence and absence of methyl esters of unsaturated fatty acids. As shown in Fig. 1, incubation of BSA, GS, or insulin with 10 mM methyl linolenate led to a substantial time-dependent increase in protein carbonyl content. After 6 hr, the carbonyl content of protein incubated in the presence of methyl linolenate was 2–3 times greater than in proteins incubated in the absence of linolenate. The ability of linolenate to stimulate carbonyl formation of BSA is almost completely dependent on the presence of ascorbate and is appreciably greater when ascorbate and Fe3+ are both present. The fact that ascorbate alone can support the linolenate-dependent increase in carbonyl formation is likely because of trace contamination of the reaction mixture by transition metals.
Figure 1.
Formation of carbonyl derivatives of proteins in the presence of methyl linolenate. Reaction mixtures (37°C) contained 2 mg/ml BSA (A), 2 mg/ml GS (B), or 1 mg/ml insulin (C). In addition, the mixtures contained 50 mM Hepes buffer, pH 7.2, and, where indicated, various supplements: 10 mM methyl linolenate (L), 25 mM ascorbate (Asc), of 0.1 mM FeCl3 (Fe). Supplements were as follows: ♦, L+Asc+Fe; ▴, L+Asc; ▵, L+Fe; ×, L; and ■, Asc+Fe; ●, Asc. For reactions with GS (B), mixtures also contained 100 mM KCl and 10 mM MgCl2.
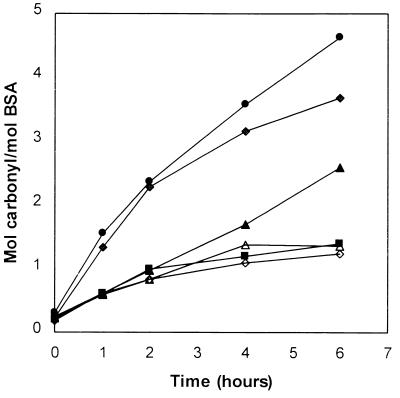
It is evident from data summarized in Fig. 2 that only polyunsaturated fatty acids are able to stimulate carbonyl formation of proteins. No increase in protein carbonyl was observed with either the saturated stearic acid (C18:0) or the monounsaturated fatty acid oleic acid (C18:1). However, methyl esters of the polyunsaturated fatty acids linoleic acid (C18:2), linolenic acid (C18:3), and arachidonic acid (C20:4) promoted carbonyl formation. Their ability to do so increases with the degree of unsaturation, in the order C18:2 < C18:3 < C20:4, which is consistent with the proposition that lipid oxidation products are involved in the enhancement of protein carbonyl formation.
Figure 2.
Lipid-dependent oxidation of BSA to carbonyl derivatives. Mixtures containing BSA (2 mg/ml), 50 mM Hepes buffer at pH 7.2, 25 mM ascorbate, 0.1 mM FeCl3, and 10 mM methyl arachidonate (●), methyl linolenate (♦), methyl linoleate (▴), methyl oleate (⋄), methyl stearate (▵), or no lipid (■) were incubated at 37°C.
Loss of lysine residues.
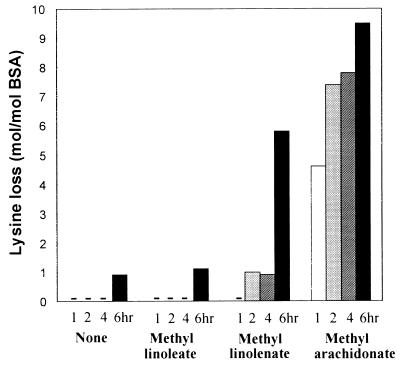
In addition to forming protein carbonyls, the exposure of BSA to polyunsaturated fatty acids in the presence of ascorbate and Fe3+ leads to loss of lysine residues. Again, the loss of lysine is strongly dependent on the degree of fatty acid unsaturation. As shown in Fig. 3, there is little or no loss of lysine residues in BSA during 6-hr incubation with methyl linoleate. However, a significant loss is induced by methyl linolenate, especially after prolonged incubation (6 hr), and, in the presence of methyl arachidonate, lysine loss increases progressively with time from a value of 4.6 mol/mol of protein after 1 hr to a value of 9.5 mol/mol after 6 hr.
Figure 3.
Loss of lysine residues after incubation of BSA with lipid and the ascorbate–iron system. BSA (2 mg/ml) was incubated with 25 mM ascorbate and 0.1 mM FeCl3 in Hepes buffer, pH 7.2, in the presence or absence of 10 mM lipid for the indicated times.
CML is a chemically well characterized advanced glycation end product of proteins (23, 26), but it has also been shown to be formed during metal-catalyzed lipid peroxidation in the presence of proteins (27). However, under our experimental conditions, only trace amounts of CML could be detected after exposing protein to metal-catalyzed oxidation for 6 hr, in either the presence or absence of lipid.
The fatty acid-dependent loss of lysine residues could reflect (i) formation of Schiff bases by direct reaction of lysine amino groups with lipid-derived aldehydes, (ii) Michael addition of the lysine amino group to the double bond of α,β-unsaturated aldehydes (e.g., 4-hydroxy-2-nonenal), which are formed during peroxidation of polyunsaturated fatty acids (1), or (iii) direct oxidation of lysine residues by LOOH or LOOH-derived radicals (LOO⋅, LO⋅). Significantly, the imidazole group of histidine and cysteine sulfhydryl groups can also undergo Michael addition reactions (1). However, we could not detect any loss of histidine residues during exposure to oxidation of proteins in the presence of lipids. The possibility that Michael addition to sulfhydryl groups is involved was not investigated.
Lipid Peroxidation.
Lipid hydroperoxides.
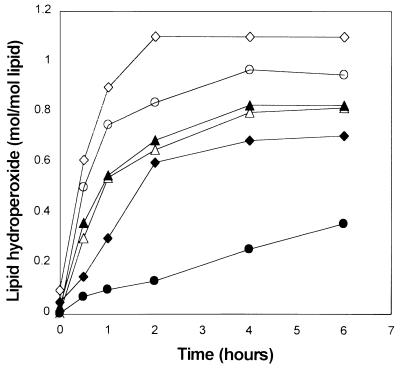
Metal-catalyzed oxidation of the methyl esters of linoleic, linolenic, and arachidonic acids led in each case to rapid formation of about 1.0 mol/mol alkyl-hydroperoxide derivative during the first 2 hr of incubation (Fig. 4). When the reactions were carried out in the presence of BSA (2 mg/ml), the yield of lipid hydroperoxide was substantially lower, by an amount that correlated with the degree of unsaturation of the lipid substrate. Thus, BSA had no effect on the level of peroxide obtained with linoleate, but the levels obtained with linolenate and arachidonate after 2-hr incubation in the presence of BSA were 13% and 35% lower, respectively, than those observed during incubation in the absence of BSA (Fig. 4).
Figure 4.
Formation of lipid hydroperoxides during ascorbate/iron-catalyzed lipid peroxidation. At a concentration of 10 mM, methyl arachidonate (●, ○), methyl linolenate (♦, ⋄), or methyl linoleate (▴, ▵) was incubated in Hepes buffer, pH 7.2, in the absence (open symbols) and in the presence (filled symbols) of BSA (2 mg/ml), for various times, as indicated.
In view of the fact that in the absence of BSA similar amounts of hydroperoxide (0.8–1.1 mol/mol) were formed by all three lipids, these results suggest either (i) that the ability of alkyl peroxides or lipid radicals to react with BSA varies with the degree of unsaturation of the fatty acids from which they are derived or (ii) that in the competition to serve as a substrate for primary reactions with metal-catalysis-generated reactive oxygen species, BSA is a better substrate than linoleate, but it is a poor substrate compared with linolenate or arachidonate.
Formation of volatile lipid aldehydes.
The amounts and composition of volatile aldehyde products formed during lipid peroxidation vary with the kind of fatty acid oxidized (Table 1). Moreover, there was a lag period of about 2 hr before significant levels of most aldehydes measured could be detected (data not shown). The levels of all volatile aldehydes in reaction mixtures containing methyl linolenate or methyl arachidonate were significantly lower when reactions were carried out in the presence of BSA (Table 1). Whereas all α,β-unsaturated aldehydes would be expected to contribute to the protein carbonyl formation by Michael addition reactions, we could detect no increase in the level of carbonyls when BSA was incubated with lipid aldehydes in the concentration (2 μM) found in the lipid oxidation mixtures. However, when tested at 2 mM concentrations, each of the α,β-unsaturated aldehydes caused a significant increase in protein carbonyl content (data not shown). Furthermore, we failed to detect 4-hydroxy-2-nonenal among the products formed in the oxidation of any one of the unsaturated fatty acids used in this study, probably because the volatility of 4-hydroxy-2-alkenals is too low for them to be isolated by headspace analysis.
Table 1.
Concentration of aldehydes formed during metal-catalyzed oxidation of lipids
| Aldehyde, mmol/mol lipid | Methyl linoleate
|
Methyl linolenate
|
Methyl arachidonate
|
|||
|---|---|---|---|---|---|---|
| −BSA | +BSA | −BSA | +BSA | −BSA | +BSA | |
| trans-2-Butanal | ND | ND | 1.70 ± 0.05 | 0.97 ± 0.06 | ND | ND |
| Pentanal | 1.3 ± 0.4 | 1.6 ± 0.2 | 0.39 ± .01 | 0.28 ± 0.05 | 9.6 ± 0.1 | 5.4 ± 0.3 |
| trans-Pentenal | ND | ND | 5.5 ± 0.1 | 4.1 ± 0.1 | ND | ND |
| Hexanal | 5.8 ± 1.3 | 4.3 ± 0.5 | ND | ND | 61 ± 3.0 | 31 ± 1.0 |
| trans-Hexenal | 0.20 ± 0.05 | 0.15 ± 0.01 | 0.86 ± 0.05 | 0.56 ± 0.04 | 0.41 ± 0.05 | 0.18 ± 0.06 |
| trans-Heptenal | 0.86 ± 0.13 | 0.62 ± 0.05 | ND | ND | 3.6 ± 0.1 | 1.4 ± 0.1 |
| trans,trans-2,4-Heptadienal | ND | ND | 1.8 ± 0.1 | 1.5 ± 0.1 | ND | ND |
| trans-Octenal | 0.36 ± 0.05 | 0.32 ± 0.05 | 0.22 ± 0.01 | 0.14 ± 0.04 | 6.7 ± 0.05 | 3.5 ± 0.05 |
| trans-2-Nonenal | ND | ND | ND | ND | 1.6 ± 0.2 | 1.0 ± 0.1 |
| trans,trans-2,4-Nonadienal | 0.36 ± 0.05 | 0.34 ± 0.04 | ND | ND | 0.84 ± 0.08 | 0.74 ± 0.02 |
| trans,trans-2,4-Decadienal | 0.64 ± 0.09 | 0.44 ± 0.13 | ND | ND | 3.7 ± 0.1 | 3.6 ± 0.1 |
Reaction mixtures containing 10 mM lipid, 25 mM ascorbate, and 0.1 mM FeCl3 were incubated at 37°C for 6 hr in the presence or absence of 2 mg/ml BSA. ND, none detected. Results are presented as mean ± SD.
The yields of pentanal, hexanal, trans-2-hexenal, trans-2-heptenal, and trans-2-octenal from arachidonic acid and of pentanal and trans-2-hexenal from linolenic acid were substantially lower when the reactions were carried out in the presence of BSA than in its absence; however, BSA had no effect on the formation of these aldehydes from linoleic acid (Table 1). The in vitro reaction of any one particular lipid aldehyde with BSA should not be affected by the structure of the lipid from which it was derived. Moreover, we observed that pentanal and hexanal (2 mM) could not promote formation of carbonyls in BSA (data not shown). Therefore, the BSA-dependent decrease in the production of various lipid aldehydes from polyunsaturated fatty acids might reflect preferential reaction of lipid hydroperoxide or lipid radical intermediates with amino acid residues of BSA. This would lead to a decrease in the amount of lipid hydroperoxides and lipid radicals that are available for further conversion to aldehydic products.
MDA formation.
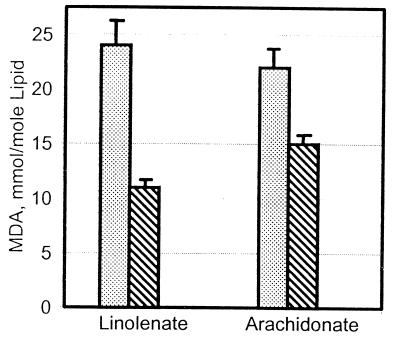
In addition to the aldehydes shown in Table 1, we also measured the levels of MDA that are formed during oxidation of linoleate, linolenate, and arachidonate. Under our conditions, MDA could not be detected among the oxidation products of methyl linoleate (results not shown), but substantial amounts (22–24 mmol/mol of lipid) were formed during the oxidation of linolenate and arachidonate (Fig. 5). Furthermore, the amount of MDA measured in reaction mixtures containing BSA was 35–50% lower than that observed in the absence of BSA (Fig. 5). This observation suggests that MDA may be an important contributor to the increase in protein carbonyl content observed during the oxidation of protein/polyunsaturated fatty acid mixtures. MDA has been shown to form reactive carbonyl derivatives of proteins (28).
Figure 5.
Conversion of lipids to MDA in the presence and absence of BSA. Reactions were carried out as described for Table 1, in the presence (hatched bars) and in the absence (gray bars) of 2 mg/ml BSA. After incubation (6 hr), the amount of MDA was measured as described in the text.
Lipid Hydroperoxide-Mediated Protein Modifications.
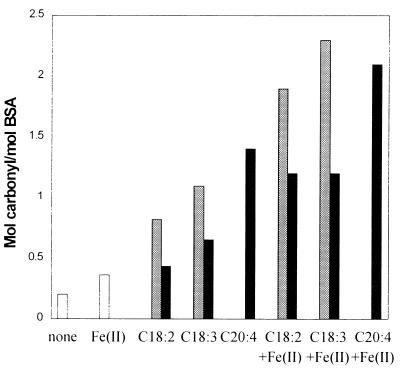
To test the possibility that lipid hydroperoxides formed during the oxidation of polyunsaturated fatty acids (Fig. 4) contribute to the generation of protein carbonyls (Figs. 1 and 2), we studied directly of the ability of lipid hydroperoxides isolated from metal-catalyzed lipid oxidation mixtures to promote formation of carbonyl groups in BSA. As shown in Fig. 6, incubation of BSA with the mixture of lipid hydroperoxides led to a substantial increase in the level of protein carbonyl groups. Significantly, hydroperoxides from linolenate were more effective in generating carbonyls than those derived from linoleate. It is also noted that carbonyl formation was greatly enhanced by further addition of Fe2+, which by itself had little, if any, effect on carbonyl formation. These results are consistent with a free radical mechanism (alkoxyl radicals) of carbonyl formation. The elevated level of carbonyl observed immediately after addition of peroxides remained unchanged after 4-hr incubation (data not shown), indicating that the reaction with lipid hydroperoxides is extremely fast and that the peroxides are extremely unstable under the conditions employed.
Figure 6.
Lipid hydroperoxide-dependent conversion of BSA to carbonyl derivatives. Reaction mixtures containing 10 mM concentrations of a chemically prepared mixture of lipid hydroperoxides (gray bars), pure lipid hydroperoxide preparations (black bars), or no hydroperoxide (open bars) were incubated with 2 mg/ml BSA in 50 mM Hepes buffer, pH 7.2, in both the presence and absence of 1 mM Fe2+. After 2 hr at 37°C, the protein carbonyl content was measured. The lipid hydroperoxide mixtures and pure lipid hydroperoxides, methyl linoleate (C18:2), methyl linolenate (C18:3), and methyl arachidonate (C20:4) were obtained as described in the text.
Data summarized in Fig. 6 show further that incubation of BSA with the linoleic- or linolenic 13(S)-hydroperoxide yielded patterns of carbonyl formation similar to those obtained with the chemically produced mixtures of lipid hydroperoxides. Again, the formation of carbonyl groups caused by the homogeneous preparations of lipid hydroperoxides was strongly dependent on the degree of unsaturation. Incubation of BSA with arachidonic acid lipid hydroperoxide (C20:4) resulted in higher levels of protein carbonyls than was observed with incubation with the 13(S)-C18:2 or C18:3 lipid hydroperoxides. Moreover, the yield of carbonyls obtained with the lipid 13(S)-hydroperoxides was only one-half that observed with the corresponding chemically produced lipid hydroperoxide mixtures, suggesting that the mixed peroxide preparation contains some more highly reactive species.
Discussion
There is abundant evidence that lipid peroxidation products (radicals, lipid hydroperoxides, and reactive aldehyde derivatives) are capable of modifying proteins, both in vivo and in vitro (6, 12, 28–31). In the present study, we compared directly, under comparable experimental conditions, the ability of the ascorbate/Fe(III)/O2 metal-catalyzed oxidation system (MCO) to promote formation of lipid aldehydes and hydroperoxide derivatives of linoleic, linolenic, and arachidonic acid methyl esters, and the abilities of these lipids to promote metal-catalyzed formation of protein carbonyl derivatives of several proteins and to produce changes in amino acid composition of BSA. We found that the oxidation of proteins by the ascorbate/iron system is greatly enhanced in the presence of polyunsaturated fatty acid methyl esters and that the ability of these lipids to stimulate protein carbonyl formation is strongly dependent upon degree of unsaturation. Thus, saturated and monounsaturated fatty acids have no detectable effect on carbonyl formation, whereas the ability of polyunsaturated fatty acids to promote carbonyl formation increases in the order linoleate < linolenate < arachidonate (Fig. 2). The metal-catalyzed oxidation of polyunsaturated fatty acids leads to the formation of several products that have been shown to form protein carbonyl derivatives with proteins. These include (i) MDA, which reacts with lysine residues of proteins to form stable carbonyl derivatives (ref. 28 and confirmed by B. S. Berlett and E.R.S., unpublished results); (ii) α,β-unsaturated aldehydes, such as 4-hydroxy-2-nonenal, which can undergo Michael addition-type reactions with the ɛ-amino group of lysine residues, the sulfhydryl group of cysteine residues, and the imidazole group of histidine residues (1–5); and (iii) lipid peroxides, which can undergo metal ion-catalyzed conversion to alkoxyl and peroxyl radicals that can react directly with side chains of some amino acid residues to form carbonyl derivatives, by mechanisms analogous to those obtained with hydrogen peroxide (32). It is noteworthy that lysine residues are among the preferred targets for reaction with all three types of lipid peroxidation products, all the more so because lysine was the only amino acid found to decrease significantly when BSA was incubated with polyunsaturated fatty acids (Fig. 3). Moreover, as with carbonyl formation, the loss of lysine residues increased as a function of the degree of fatty acid unsaturation. Significantly, in addition to lysine residues, the sulfhydryl groups of proteins and the imidazole groups of histidine residues can also undergo Michael addition reactions to form protein carbonyl derivatives (2). However, Michael addition of histidine residues cannot play a major role in the observed increase in lipid-dependent formation of protein carbonyl groups in BSA, since the loss of histidine residues in the presence and absence of lipids was almost the same. The possibility that Michael additions to sulfhydryl groups of BSA are involved was not investigated.
In addition to reactions that yield carbonyl derivatives, lysine residues of proteins can react directly with lipid-derived aldehydes to form Schiff base derivatives (30). At first glance, formation of Schiff base derivatives might explain why the yields of some of the aldehydes produced by metal-catalyzed oxidation of methyl linolenate and methyl arachidonate are considerably lower in the reaction mixtures containing BSA than in its absence (Table 1) and might also account for the loss of lysine residues associated with lipid peroxidation (Fig. 3). However, neither Schiff base formation alone nor simple Michael addition reactions with lysine residues can account for the observed loss of lysine residues, since both are very sensitive to acid hydrolysis (25, 30, 31, 33) and would be destroyed during protein hydrolysis before amino acid analysis. Moreover, treatment of reaction mixtures with sodium cyanoborohydride at pH 6.0, conditions known to reduce Schiff bases to stable derivatives, did not lead to a detectable increase in the amount of lysine lost (data not shown). Therefore, unless they are followed by secondary reactions—i.e., rearrangements or polymerization reactions (34)—it is unlikely that formation of Schiff bases or Michael addition reactions contribute significantly to the observed loss of lysine residues.
We could not detect any formation of 4-hydroxy-2-nonenal in the model systems, probably because of low volatility of this hydroxy compound (Table 1). Furthermore, only (n − 6) polyunsaturated fatty acids, such as linoleic and arachidonic acids but not the (n − 3) linolenic acid, can yield 4-hydroxy-2-nonenal (35). This indicates that 4-hydroxy-2-nonenal did not cause the observed protein modifications. We concluded that formation of CML did not contribute significantly to the polyunsaturated fatty acid-dependent loss of lysine residues. Other studies have also demonstrated that CML is formed at the low mmol/mol lysine residues level (27, 36).
It is well known that metal-catalyzed oxidation of polyunsaturated lipids leads to formation of lipid hydroperoxides (9). The observation that the level of lipid hydroperoxide formed in the absence of protein is substantially greater than in the presence of protein (Fig. 4) indicates that the lipid hydroperoxides may react further with proteins. The dependence on the type of fatty acid and the presence of protein for the formation of pentanal, hexanal, trans-2-hexenal, trans-2-heptenal, and trans-2-octenal (Table 1) also suggest involvement of lipid hydroperoxides or lipid radicals in the observed protein modifications. This invites speculation that alkoxyl radicals formed in the Fe2+-dependent heterolytic cleavage of lipid hydroperoxides (37) are involved in the generation of protein derivatives (Fig. 6) and might involve site-specific interactions with lysine residues, in analogy to site-specific modifications of proteins as occurs with H2O2 metal-catalyzed oxidation systems (21, 32).
We assume that the ability of BSA to suppress the formation of lipid hydroperoxides and lipid aldehydes reflects reaction of alkoxyl radicals formed from lipid hydroperoxides with the amino acid residues in the protein. However, the possibility that the lower level of lipid oxidation products reflects competition between lipids and proteins for reactive oxygen species generated by the metal-catalyzed system cannot be ruled out.
The results presented here confirm and extend findings of other workers (11–13) demonstrating that primary lipid peroxidation products can contribute significantly to the oxidative damage of proteins as occurs under conditions of oxidative stress and in aging and age-related diseases (see ref. 38 for review).
Acknowledgments
We acknowledge the technical assistance of Inge Holmberg and the help of Barbara S. Berlett in measuring amino acid composition. We thank Dr. Benny Jensen, Danish Institute for Fisheries Research, for many fruitful suggestions and discussions throughout this study. H.H.F.R. was supported by a grant from the Danish Research and Development Program for Food Technology.
Abbreviations
- CML
Nɛ-(carboxymethyl)lysine
- GS
glutamine synthetase
- MDA
malondialdehyde
- LOOH
lipid hydroperoxide
References
- 1.Esterbauer H, Schaur R J, Zollner H. Free Radical Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Uchida K, Stadtman E R. Proc Natl Acad Sci USA. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayre L M, Arora P K, Iyer R S, Salomon R G. Chem Res Toxicol. 1993;6:19–22. doi: 10.1021/tx00031a002. [DOI] [PubMed] [Google Scholar]
- 4.Friguet B, Szweda L I, Stadtman E R. Arch Biochem Biophys. 1994;311:168–173. doi: 10.1006/abbi.1994.1222. [DOI] [PubMed] [Google Scholar]
- 5.Brunner B A, Jones A D, German J B. Chem Res Toxicol. 1995;8:552–559. doi: 10.1021/tx00046a009. [DOI] [PubMed] [Google Scholar]
- 6.Tsai L, Szweda P A, Vinogradova O, Szweda L I. Proc Natl Acad Sci USA. 1998;95:7975–7980. doi: 10.1073/pnas.95.14.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page S, Fisher C, Baumgarther B, Haas M, Kreusel U, Loidl G, Hayn M, Ziegler-Heitbrock H W, Neumeier D, Brand K. J Biol Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. J Biol Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 9.Gardner H W. Free Radical Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 10.Girotti A W. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 11.Fruebis J, Parthasarathy S, Steinberg D. Proc Natl Acad Sci USA. 1992;89:10588–10592. doi: 10.1073/pnas.89.22.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J G, Sabbagh F, Santanam N, Wilcox J N, Medford R M, Parthasarathy S. Free Radical Biol Med. 1997;23:251–259. doi: 10.1016/s0891-5849(96)00615-6. [DOI] [PubMed] [Google Scholar]
- 13.Bielicki J K, Forte T M. J Lipid Res. 1999;40:948–954. [PubMed] [Google Scholar]
- 14.Tien M, Berlett B S, Levine R L, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1999;96:7809–7814. doi: 10.1073/pnas.96.14.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller R E, Shelton E, Stadtman E R. Arch Biochem Biophys. 1974;163:155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- 16.Reeder B J, Wilson M T. Biochem J. 1998;330:1317–1323. doi: 10.1042/bj3301317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine R L. J Biol Chem. 1983;258:11828–11833. [PubMed] [Google Scholar]
- 18.Climent I, Tsai L, Levine R L. Anal Biochem. 1989;182:226–232. doi: 10.1016/0003-2697(89)90584-8. [DOI] [PubMed] [Google Scholar]
- 19.Lenz A G, Costabel U, Shaltiel S, Levine R L. Anal Biochem. 1989;177:419–425. doi: 10.1016/0003-2697(89)90077-8. [DOI] [PubMed] [Google Scholar]
- 20.Levine R L, Garland D, Oliver C N, Amici A, Climent I, Lenz A, Ahn B W, Shaltiel S, Stadtman E R. Methods Enzymol. 1990;186:464–485. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 21.Rivett A J, Levine R L. Arch Biochem Biophys. 1990;278:26–34. doi: 10.1016/0003-9861(90)90226-o. [DOI] [PubMed] [Google Scholar]
- 22.Levine R L, Williams J A, Stadtman E R, Schacter E. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 23.Knecht K J, Dunn J A, McFarland K F, McCance D R, Lyons T J, Thorpe S R, Baynes J W. Diabetes. 1991;40:190–196. doi: 10.2337/diab.40.2.190. [DOI] [PubMed] [Google Scholar]
- 24.Asakawa T, Matsushita S. Lipids. 1980;15:965–967. [Google Scholar]
- 25.Yeo H C, Helbock H J, Chyu D W, Ames B N. Anal Biochem. 1994;220:391–396. doi: 10.1006/abio.1994.1355. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed M, Thorpe S R, Baynes J W. J Biol Chem. 1986;261:889–4894. [PubMed] [Google Scholar]
- 27.Fu M-X, Requena J R, Jenkins A J, Lyons T J, Baynes J W, Thorpe S R. J Biol Chem. 1996;271:20406–20414. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 28.Burcham P C, Kuhan Y T. Biochem Biophys Res Commun. 1996;220:996–1001. doi: 10.1006/bbrc.1996.0521. [DOI] [PubMed] [Google Scholar]
- 29.Belkner J, Wiesner R, Rathman J, Sigal E, Kuhn H. Eur J Biochem. 1993;213:251–261. doi: 10.1111/j.1432-1033.1993.tb17755.x. [DOI] [PubMed] [Google Scholar]
- 30.Jürgens G, Lang J, Esterbauer H. Biochim Biophys Acta. 1986;875:103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- 31.Kato Y, Mori Y, Makino Y, Morimitsu Y, Hiroi S, Ishikawa T, Osawa T. J Biol Chem. 1999;274:20406–20414. doi: 10.1074/jbc.274.29.20406. [DOI] [PubMed] [Google Scholar]
- 32.Kato Y, Uchida K, Kawakishi S. J Biol Chem. 1992;267:23646–23651. [PubMed] [Google Scholar]
- 33.Uchida K, Sakai K, Itakura K, Osawa T, Toyokuni S. Arch Biochem Biophys. 1997;346:45–52. doi: 10.1006/abbi.1997.0266. [DOI] [PubMed] [Google Scholar]
- 34.Cohn J A, Tsai L, Friguet B, Szweda L I. Arch Biochem Biophys. 1996;328:158–164. doi: 10.1006/abbi.1996.0156. [DOI] [PubMed] [Google Scholar]
- 35.Pryor W A, Porter N A. Free Radical Res Commun. 1990;8:541–543. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- 36.Anderson M M, Requena J R, Crowley J R, Thorpe S R, Heinecke J W. J Clin Invest. 1999;104:103–113. doi: 10.1172/JCI3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies M J, Slater T F. Biochem J. 1987;245:167–173. doi: 10.1042/bj2450167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadtman E R, Berlett B S. In: Reactive Oxygen Species in Biological Systems. Gilbert D L, Colton C A, editors. New York: Kluwer Academic/Plenum Publishers; 1999. pp. 657–675. [Google Scholar]