Abstract
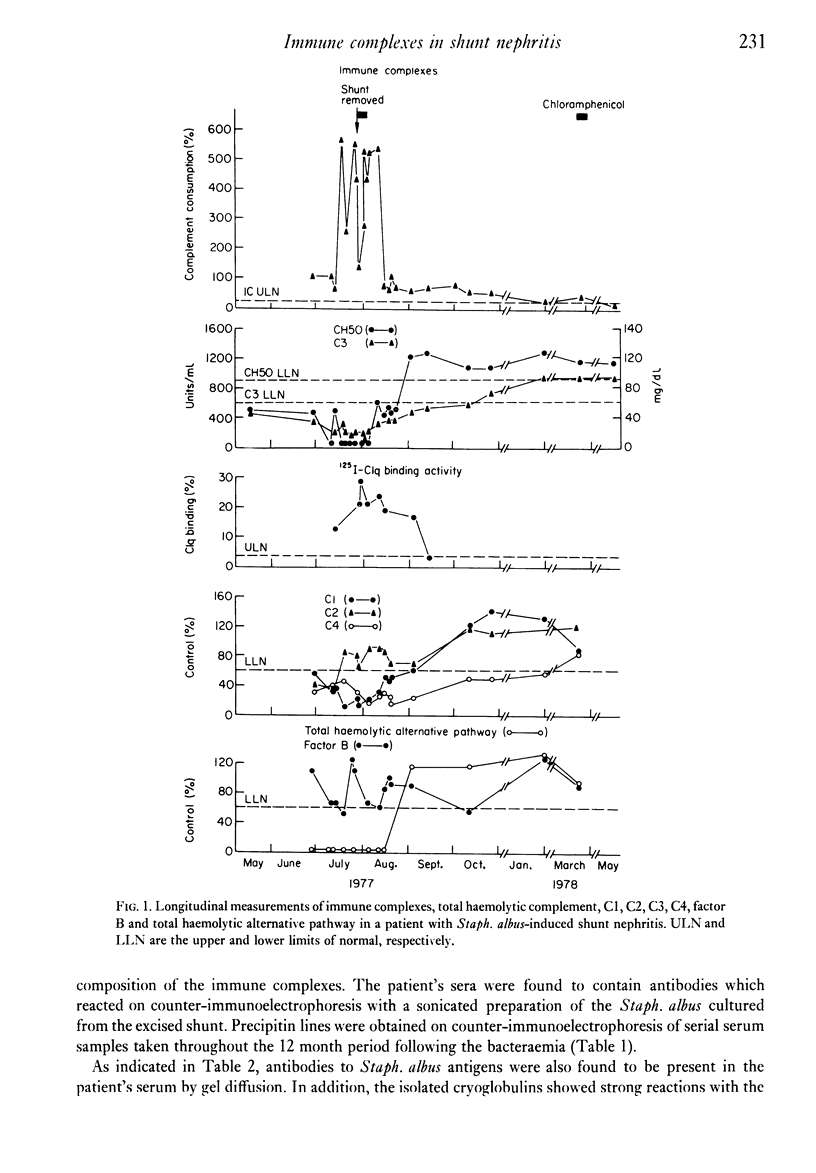
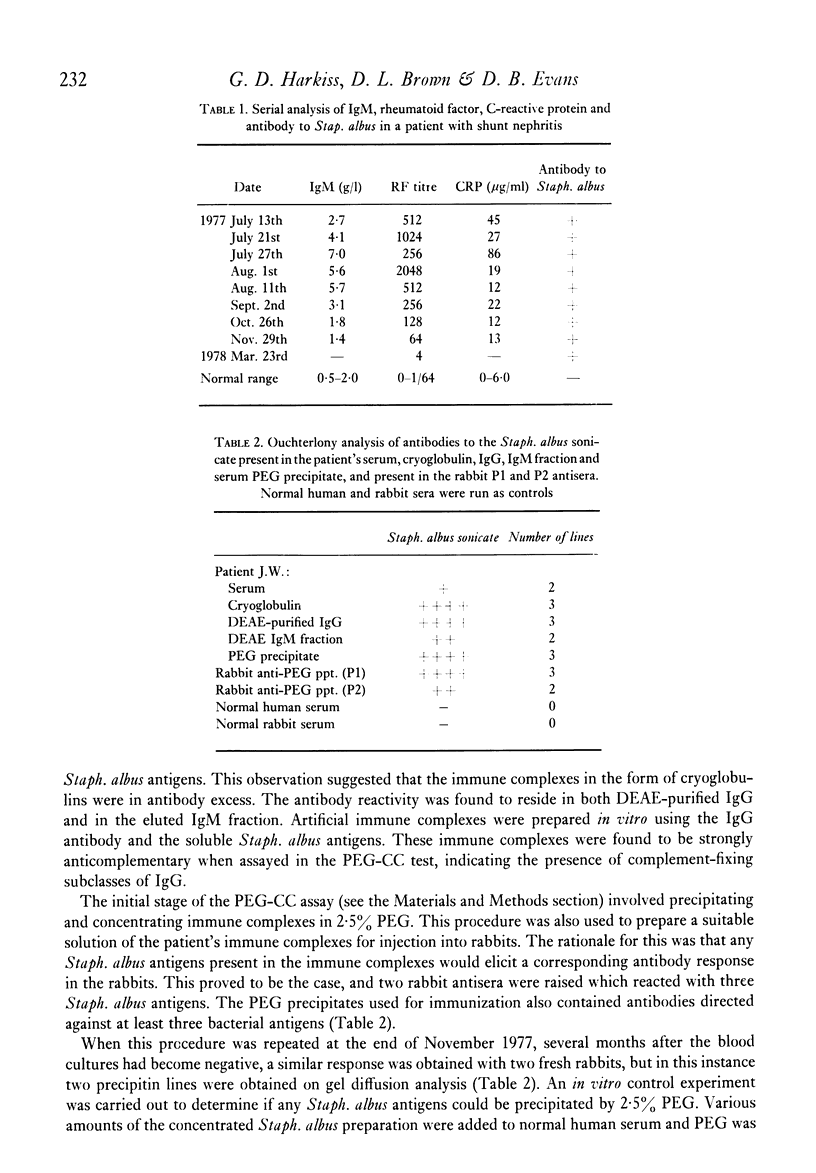
The direct measurement and partial characterization of circulating immune complexes has been performed in a longitudinal study of a patient with Staphylococcus albus-induced shunt nephritis. The high levels of immune complexes were associated with cryoglobulinaemia and hypocomplementaemia. The activation of complement was found to be via the classical pathway, but the functioning of the alternative pathway may have been impaired in vivo due to very low levels of C3. The host response to the infection was also characterized by the production of a marked macroglobulinaemia, high titres of rheumatoid factor and a typical acute phase increase in the C-reactive protein level. Immune complex levels were persistently elevated many months after the removal of the focus of the infection. A possible explanation for this surprising finding may lie in the nature of the antigens in the immune complexes. It was found that the immune complexes contained both antibodies to and antigens from Staphlococcus albus. In particular, glycerol teichoic acid and staphylococcal nuclease were identified as components of the immune complexes present during the acute phase. Glycerol teichoic acid was also identified in the immune complexes found later although other Staphylococcus albus antigens as yet unidentified were also present and persisted in the circulation for several months.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton W. K., Sande M. A., Normansell D. E., Sturgill B. C., Westervelt F. B., Jr Ventriculojugular shunt nephritis with Corynebacterium bovis. Successful therapy with antibiotics. Am J Med. 1975 Sep;59(3):417–423. doi: 10.1016/0002-9343(75)90401-5. [DOI] [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus D. R., Siegel J., Petras K., Skor D., Osmand A. P., Gewurz H. Complement activation by interaction of polyanions and polycations. III. Complement activation by interaction of multiple polyanious and polycations is the presence of C-reactive protein. J Immunol. 1977 Jan;118(1):83–87. [PubMed] [Google Scholar]
- Ekstedt R. D. Immune response to surface antigens of Staphylococcus aureus and their role in resistance to staphylococcal disease. Ann N Y Acad Sci. 1974 Jul 31;236(0):203–220. doi: 10.1111/j.1749-6632.1974.tb41492.x. [DOI] [PubMed] [Google Scholar]
- FELTON L. D., PRESCOTT B., KAUFFMANN G., OTTINGER B. Pneumococcal antigenic polysaccharide substances from animal tissues. J Immunol. 1955 Mar;74(3):205–213. [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974 May;112(5):1939–1948. [PubMed] [Google Scholar]
- Harkiss G. D., Brown D. L. Detection of immune complexes by a new assay, the polyethylene glycol precipitation-complement consumption test (PEG-CC). Clin Exp Immunol. 1979 Apr;36(1):117–129. [PMC free article] [PubMed] [Google Scholar]
- Holt R. The classification of staphylococci from colonized ventriculo-atrial shunts. J Clin Pathol. 1969 Jul;22(4):475–482. doi: 10.1136/jcp.22.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Elson J., Christie G. H., Kinsky R. G. Studies on immunological paralysis. II. The detection and significance of antibod-forming cells in the spleen during immunological paralysis with type 3 pneumococcal polysaccharide. Clin Exp Immunol. 1969 Jan;4(1):41–53. [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Humphrey J. H. Synthetic antigens composed exclusively of L- or D- amino acids. II. Effect of optical configuration on the metabolism and fate of synthetic polypeptide antigens in mice. Immunology. 1968 Feb;14(2):225–234. [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. B., McIntosh R. The pathogenesis of the renal lesion in a patient with streptococcal disease, infected ventriculoatrial shunt, cryogobulinemia and nephritis. Am J Med. 1971 Feb;50(2):262–268. doi: 10.1016/0002-9343(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Moncrieff M. W., Glasgow E. F., Arthur L. J., Hargreaves H. M. Glomerulonephritis associated with Staphylococcus albus in a Spitz Holter valve. Arch Dis Child. 1973 Jan;48(1):69–72. doi: 10.1136/adc.48.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters W., Mussche M., Becaus I., Ringoir S. Shunt nephritis. Clin Nephrol. 1978 Mar;9(3):122–125. [PubMed] [Google Scholar]
- Rames L., Wise B., Goodman J. R., Piel C. F. Renal disease with Staphylococcus albus bacteremia. A complication in ventriculoatrial shunts. JAMA. 1970 Jun 8;212(10):1671–1677. [PubMed] [Google Scholar]
- Reeder W. J., Ekstedt R. D. Study of the interaction of concanavalin A with staphylocccal teichoic acids. J Immunol. 1971 Feb;106(2):334–340. [PubMed] [Google Scholar]
- Schoenbaum S. C., Gardner P., Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis. 1975 May;131(5):543–552. doi: 10.1093/infdis/131.5.543. [DOI] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Processing of streptococcal cell walls by rat macrophages and human monocytes in vitro. Infect Immun. 1977 Sep;17(3):591–598. doi: 10.1128/iai.17.3.591-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickler G. B., Shin M. H., Burke E. C., Holley K. E., Miller R. H., Segar W. E. Diffuse glomerulonephritis associated with infected ventriculoatrial shunt. N Engl J Med. 1968 Nov 14;279(20):1077–1082. doi: 10.1056/NEJM196811142792003. [DOI] [PubMed] [Google Scholar]
- Strife C. F., McDonald B. M., Ruley E. J., McAdams A. J., West C. D. Shunt nephritis: the nature of the serum cryoglobulins and their relation to the complement profile. J Pediatr. 1976 Mar;88(3):403–413. doi: 10.1016/s0022-3476(76)80254-5. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Wadström T. Biological properties of extracellular proteins from staphylococcus. Ann N Y Acad Sci. 1974 Jul 31;236(0):343–361. doi: 10.1111/j.1749-6632.1974.tb41502.x. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Dukor P. The role of lysosomes in immune responses. Adv Immunol. 1970;12:283–331. doi: 10.1016/s0065-2776(08)60172-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J Immunol. 1978 Jan;120(1):174–178. [PubMed] [Google Scholar]
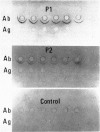
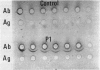
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]