Abstract
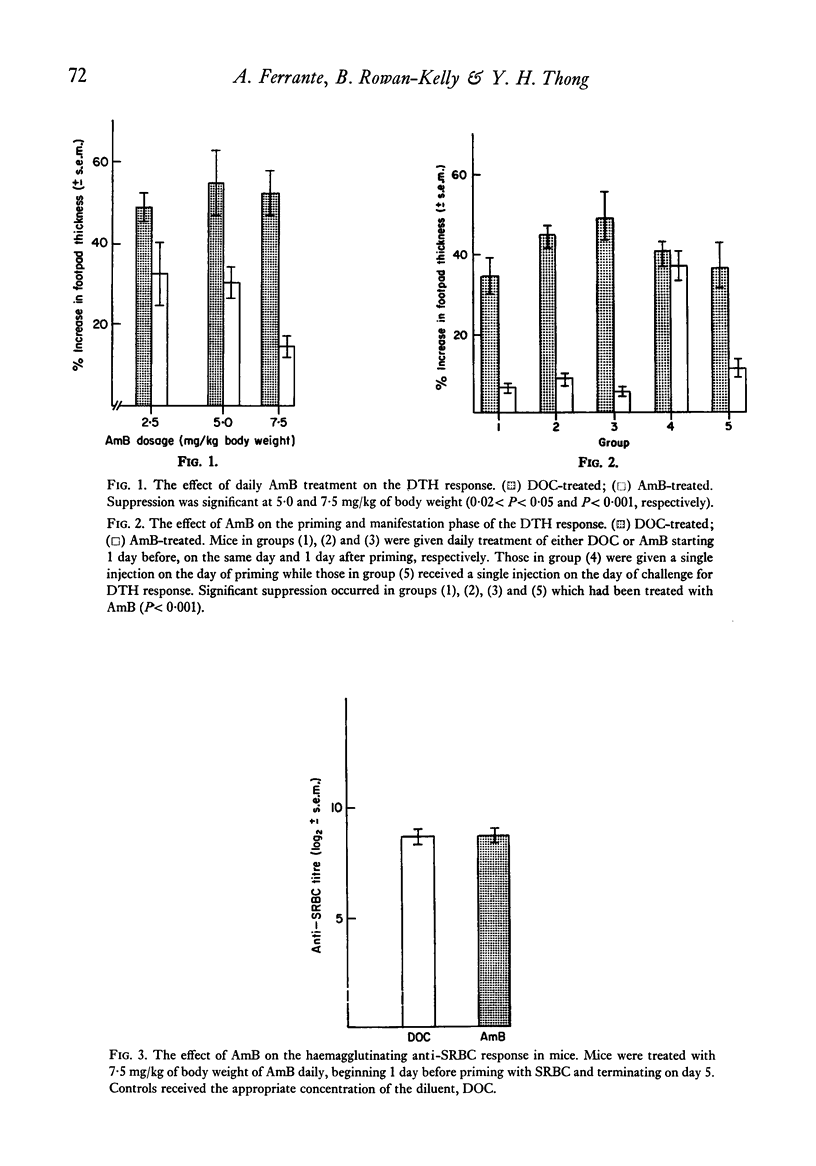
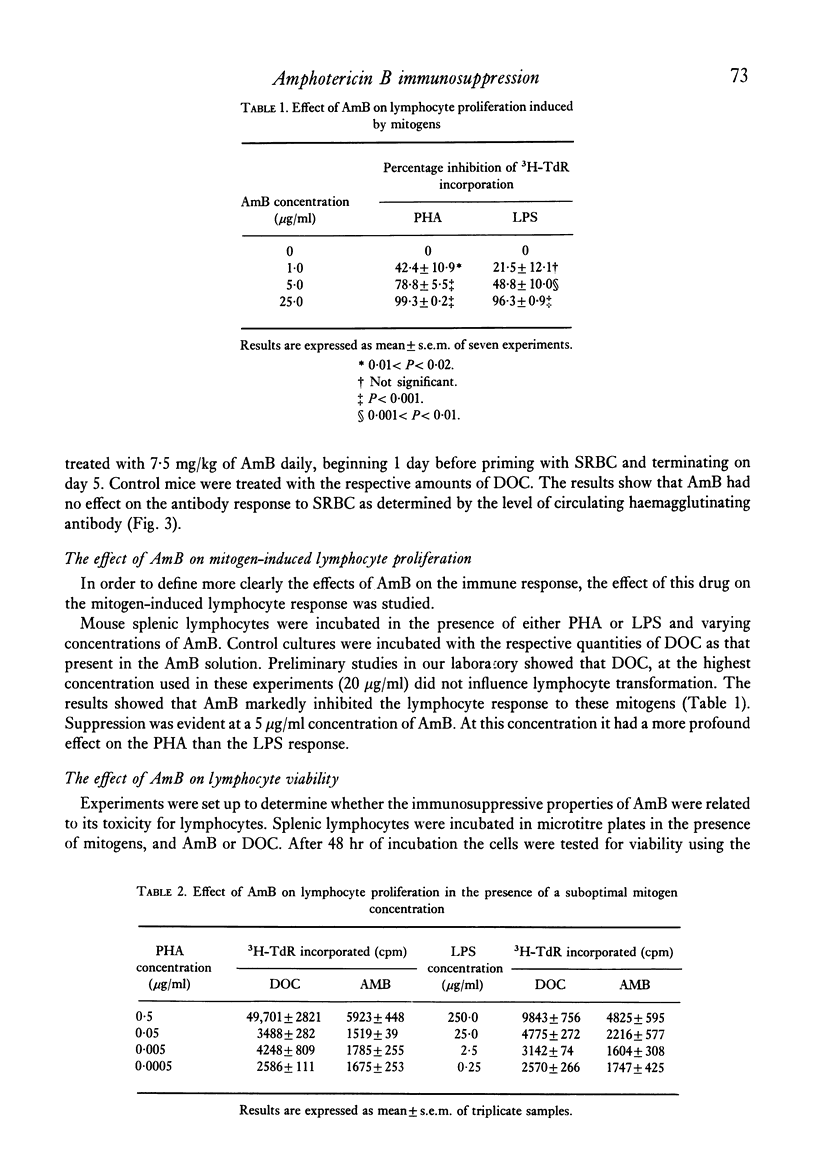
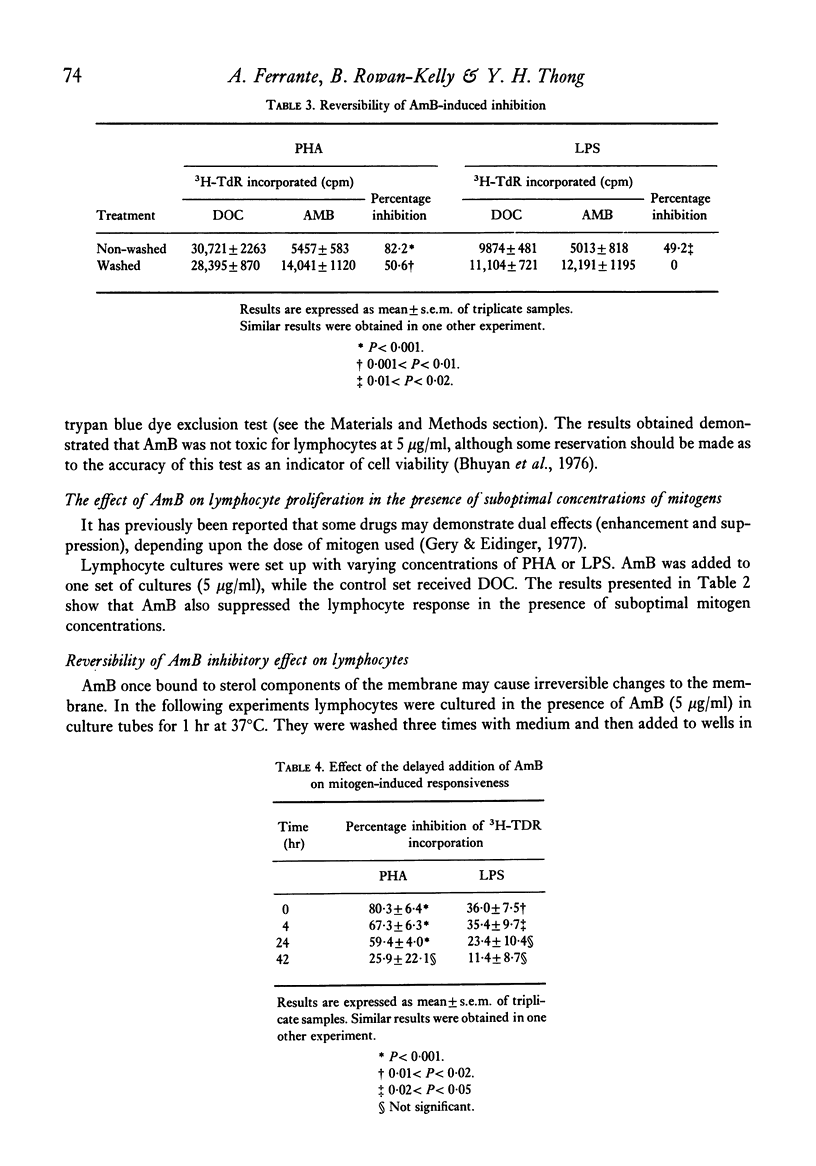
The polyene antibiotic amphotericin B (AmB) caused a marked suppression of the cell-mediated immune response in mice. Similar treatment did not effect the humoral antibody response. The immunosuppressive property of the drug was related to its ability to inhibit the manifestation rather than the induction phase of the delayed-type hypersensitivity response. In vitro AmB suppressed mitogen-induced lymphocyte proliferation. The drug seemed to act at the proliferative phase of the response. Results presented show that the T cell response was much more sensitive to the action of AmB than was the B cell response. During AmB chemotherapy consideration must be given to the immunosuppressive properties of this drug.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. E. Chemotherapy of systemic mycoses (first of two parts). N Engl J Med. 1974 Jan 3;290(1):30–32. doi: 10.1056/NEJM197401032900107. [DOI] [PubMed] [Google Scholar]
- Bhuyan B. K., Loughman B. E., Fraser T. J., Day K. J. Comparison of different methods of determining cell viability after exposure to cytotoxic compounds. Exp Cell Res. 1976 Feb;97(2):275–280. doi: 10.1016/0014-4827(76)90617-0. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Ray C., Quie P. G. Inhibition of human neutrophil chemotaxis and chemiluminescence by amphotericin B. Infect Immun. 1976 Jul;14(1):315–317. doi: 10.1128/iai.14.1.315-317.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke T. J., Little J. R., Shirley S. F., Lynch R. G. Augmentation of murine immune responses by amphotericin B. Cell Immunol. 1977 Sep;33(1):180–190. doi: 10.1016/0008-8749(77)90145-9. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Gentry L. O., Remington J. S. Resistance against Cryptococcus conferred by intracellular bacteria and protozoa. J Infect Dis. 1971 Jan;123(1):22–31. doi: 10.1093/infdis/123.1.22. [DOI] [PubMed] [Google Scholar]
- Gery I., Eidinger D. Selective and opposing effects of cytochalasin B and other drugs on lymphocyte responses to different doses of mitogens. Cell Immunol. 1977 Apr;30(1):147–155. doi: 10.1016/0008-8749(77)90055-7. [DOI] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity. III. Effect of cyclophosphamide on the suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):172–176. doi: 10.1002/eji.1830080306. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Taylor R. L. Host defense in cryptococcosis. I. An in vivo model for evaluating immune response. Int Arch Allergy Appl Immunol. 1978;57(2):101–113. [PubMed] [Google Scholar]
- Howard D. H., Otto V. Experiments on lymphocyte-mediated cellular immunity in murine histoplasmosis. Infect Immun. 1977 Apr;16(1):226–231. doi: 10.1128/iai.16.1.226-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Narimatsu H., Saito K. Adjuvant effect of nystatin on in vitro antibody response of mouse spleen cells to heterologous erythrocytes. Cell Immunol. 1975 May;17(1):300–305. doi: 10.1016/s0008-8749(75)80030-x. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of delayed-type hypersensitivity. I. T suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1977 Oct;7(10):714–718. doi: 10.1002/eji.1830071013. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Kostiala A. A. Role of Lymphocytes in Cellular resistance in infection. Contemp Top Immunobiol. 1976;5:237–266. [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Tewari R. P., Sharma D., Solotorovsky M., Lafemina R., Balint J. Adoptive transfer of immunity from mice immunized with ribosomes or live yeast cells of Histoplasma capsulatum. Infect Immun. 1977 Mar;15(3):789–795. doi: 10.1128/iai.15.3.789-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A. Inhibition of mitogen-induced human lymphocyte proliferative responses by tetracycline analogues. Clin Exp Immunol. 1979 Mar;35(3):443–446. [PMC free article] [PubMed] [Google Scholar]
- Thong Y. H., Ness D. Antifungal drugs and neutrophil chemotaxis. Lancet. 1977 Sep 10;2(8037):568–568. doi: 10.1016/s0140-6736(77)90717-6. [DOI] [PubMed] [Google Scholar]
- Tärnvik A., Ansehn S. Effect of amphotericin B and clotrimazole on lymphocyte stimulation. Antimicrob Agents Chemother. 1974 Nov;6(5):529–533. doi: 10.1128/aac.6.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]


