Abstract
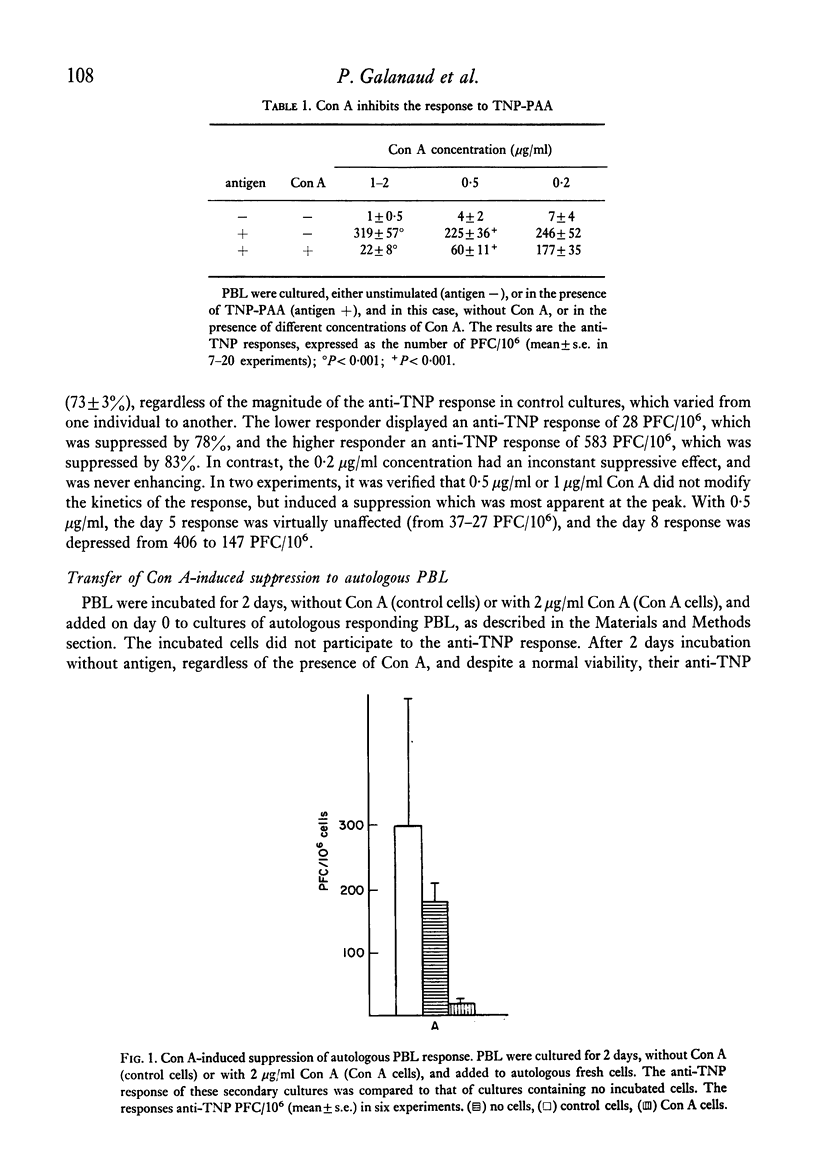
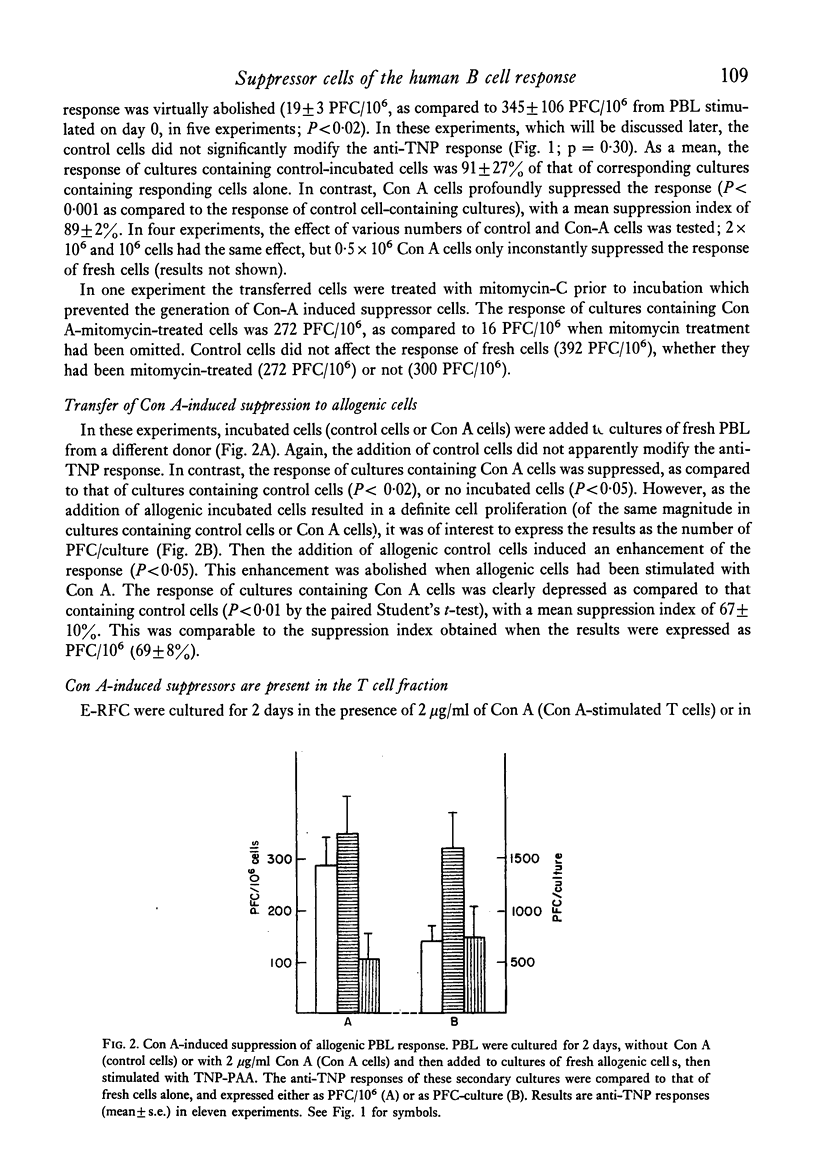
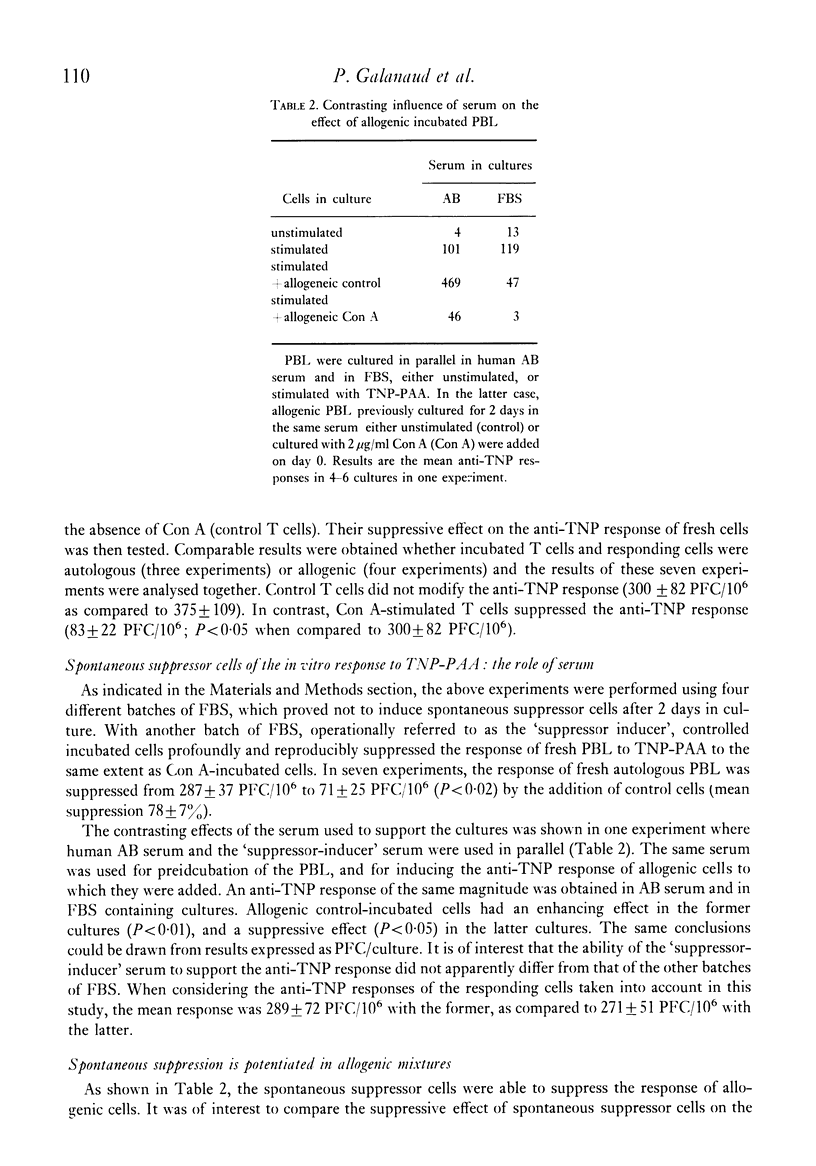
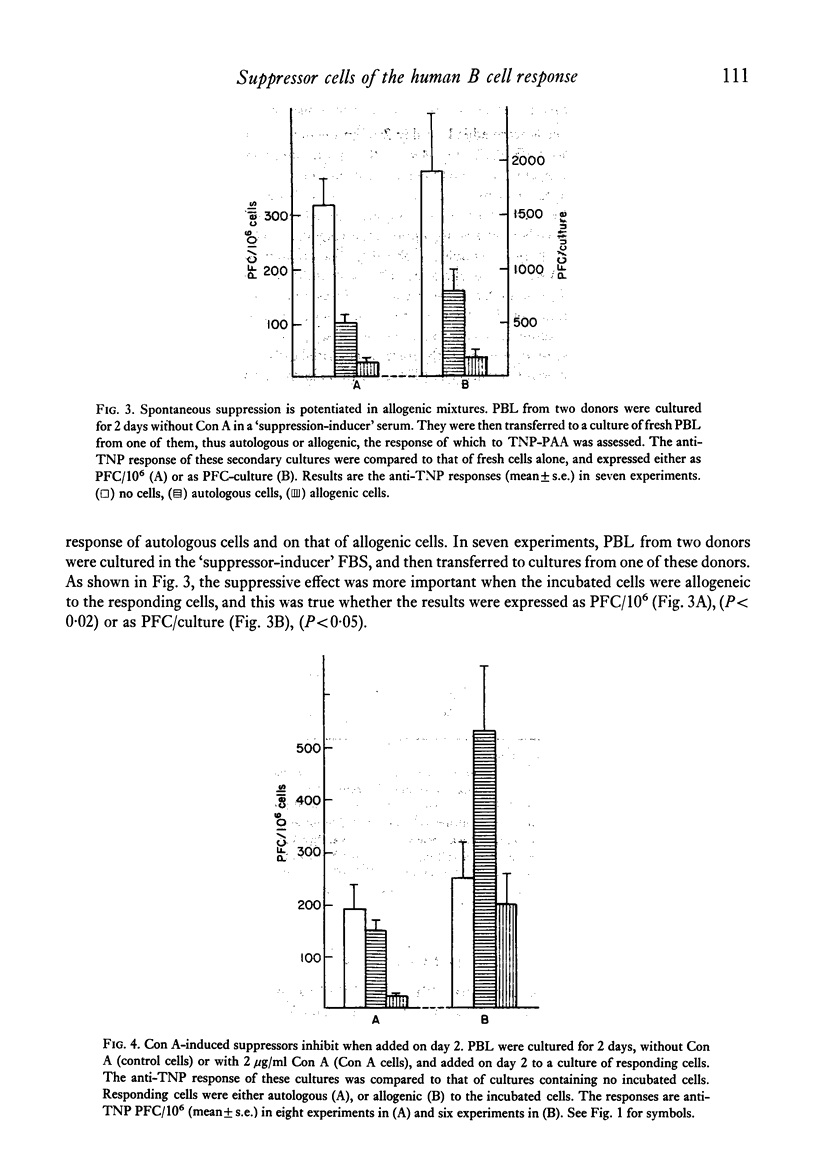
The specific response of human peripheral blood lymphocyte cultures to TNP-polyacrylamide was suppressed by the addition of concanavalin A (Con A). A dose of Con A (0.5 microgram/ml) could be selected, which induced a reproducible but incomplete suppression independent of the magnitude of the anti-TNP response. Con A-stimulated cells could transfer the suppression to autologous or allogeneic responding cells. The suppressor activity was present in the E-rosette forming cell fraction and was abolished by mitomycin C treatment prior to incubation. With a particular batch of foetal bovine serum, spontaneous suppressor cells were produced which suppressed the response of autologous and allogeneic lymphocytes. In allogenic mixtures, the otherwise enhancing allogeneic effect was replaced by a marked suppression from spontaneous suppressor cells. This suppression was higher than that exerted on autologous lymphocytes, suggesting that an unexpected negative allogenic effect had taken place. Spontaneous suppressors were ineffective when added on day 2 of a culture responding to TNP-polyacrylamide, whereas Con A induced suppressors were fully effective.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F. Evaluation of T-cells and thymic serum factors in man using the rosette technique. Transplant Rev. 1973;16(0):196–217. doi: 10.1111/j.1600-065x.1973.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Burns F. D., Marrack P. C., Kappler J. W., Janeway C. A., Jr Functional heterogeneity among the T-derived lymphocytes of the mouse. IV. Nature of spontaneously induced suppressor cells. J Immunol. 1975 Apr;114(4):1345–1347. [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Regulation of cellular and humoral immune responses by T-cell subclasses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):23–32. doi: 10.1101/sqb.1977.041.01.006. [DOI] [PubMed] [Google Scholar]
- Delfraissy J. F., Galanaud P., Dormont J., Wallon C. Primary in vitro antibody response from human peripheral blood lymphocytes. J Immunol. 1977 Feb;118(2):630–635. [PubMed] [Google Scholar]
- Delfraissy J. F., Galanaud P., Dormont J., Wallon C. Primary in vitro antibody response of human peripheral blood lymphocytes: role of phagocytic mononuclear cells. J Immunol. 1978 Apr;120(4):1283–1288. [PubMed] [Google Scholar]
- Dosch H-M, Gelfand E. W. Generation of human plaque-forming cells in culture: tissue distribution, antigenic and cellular requirements. J Immunol. 1977 Jan;118(1):302–308. [PubMed] [Google Scholar]
- Dugan E., Niederhuber J. E., Frelinger J. A., Mayo L., Shreffler D. C. Con A induced suppressor cells: suppression with I region incompatibility. Cell Immunol. 1977 Mar 15;29(2):322–330. doi: 10.1016/0008-8749(77)90326-4. [DOI] [PubMed] [Google Scholar]
- Dutton R. W. Suppressor T cells. Transplant Rev. 1975;26:39–55. doi: 10.1111/j.1600-065x.1975.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Greaves M. F., Parker D. C., Rittenberg M. B. Direct triggering of B lymphocytes by insolubilized antigen. Eur J Immunol. 1974 Sep;4(9):591–597. doi: 10.1002/eji.1830040903. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. V. Kinetics and mechanisms of suppression of plaque-forming cell responses by concanavalin A-generated suppressor cells. J Immunol. 1978 Mar;120(3):700–708. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. X. Heterogeneity of concanavalin A-generated suppressor cells of the pokeweed mitogen-induced plaque-forming cell response of human peripheral blood lymphocytes. J Immunol. 1978 Aug;121(2):559–565. [PubMed] [Google Scholar]
- Hayward A. R., Layward L., Lydyard P. M., Moretta L., Dagg M., Lawton A. R. Fc-receptor heterogeneity of human suppressor T cells. J Immunol. 1978 Jul;121(1):1–5. [PubMed] [Google Scholar]
- Jandinski J., Cantor H., Tadakuma T., Peavy D. L., Pierce C. W. Separation of helper T cells from suppressor T cells expressing different Ly components. I. Polyclonal activation: suppressor and helper activities are inherent properties of distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1382–1390. doi: 10.1084/jem.143.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ginsburg W. W., Finkelman F. D., Ziff M. Control of human B lymphocyte responsiveness: enhanced suppressor T cell activity after in vitro incubation. J Immunol. 1978 Mar;120(3):902–910. [PubMed] [Google Scholar]
- Luzzati A. L., Hengartner H., Schreier M. H. Induction of plaque-forming cells in cultured human lymphocytes by combined action of antigen and EB virus. Nature. 1977 Sep 29;269(5627):419–420. doi: 10.1038/269419a0. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespoli L., Möller G., Waterfield D., Ekstedt R. Experimental conditions for obtaining suppressor and helper effects on the primary in vitro immune response by lymphocytes activated by polyclonal T-cell activators. J Exp Med. 1977 Mar 1;145(3):631–643. doi: 10.1084/jem.145.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R. Appearance of non-specific suppressor T cells during in vitro culture. Immunology. 1977 Oct;33(4):597–603. [PMC free article] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Shou L., Good R. A., Choi Y. S. Suppression of immunoglobulin synthesis and secretion by peripheral blood lymphocytes from normal donors. Proc Natl Acad Sci U S A. 1977 May;74(5):2099–2103. doi: 10.1073/pnas.74.5.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore A., Dosch H., Gelfand E. W. Induction and separation of antigen-dependent T helper and T suppressor cells in man. Nature. 1978 Aug 10;274(5671):586–587. doi: 10.1038/274586a0. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Swain S. L., Dutton R. W. Negative allogeneic effects in vitro. I. Allogeneic T cells markedly suppress the secondary antibody-forming cell response. J Immunol. 1977 Jun;118(6):2262–2268. [PubMed] [Google Scholar]
- Tse H. Y., Dutton R. W. Separation of helper and suppressor T lymphocytes. III. Positive and negative effects of mixed lymphocyte reaction-activated T cells. J Immunol. 1978 Apr;120(4):1149–1152. [PubMed] [Google Scholar]
- UytdeHaag F., Heynen C. J., Ballieux R. E. Induction of antigen-specific human suppressor T lymphocytes in vitro. Nature. 1978 Feb 9;271(5645):556–557. doi: 10.1038/271556a0. [DOI] [PubMed] [Google Scholar]


