Abstract
In strains of mice with high or low sensitivity to androgen, previous studies demonstrated that sex differences in the levels of certain Igs occurred only in the high androgen responder (HAR) strain, but not in the low androgen responder (LAR) strain. HAR C57L/J males, whether intact or castrated, had lower levels of IgM and IgG2 than C57L/J females, and lower levels of IgM and IgG2 than LAR A/J mice (Cohn & Hamilton, 1976). The current study indicates that the pattern of low immune response characteristic of HAR males in non-specific responses also occurs in response to three different antigens and is evident in another HAR strain, the RF/J.
Results show that in response to type 3 pneumococcal polysaccharide (SIII), HAR C57L/J males consistently produced lower levels of antibody than C57L/J females, whereas LAR A/J males and females produced similar levels of anti-SIII. HAR C57L/J males also produced significantly lower levels of anti-SIII than LAR A/J males following restricted doses of SIII (50 ng). Altering the concentration of circulating testosterone in HAR C57L/J males had no effect on their anti-SIII responses. In assays of another pair of HAR and LAR strains, the RF/J and 129/J, HAR RF/Js had significantly lower anti-SIII responses than LAR 129/Js. In response to bovine serum albumin (BSA), HAR C57L/J mice showed sex differences in anti-BSA responses both 14 and 21 days after immunization, whereas HAR A/J mice showed sex differences on day 14 only, before peak responses developed. Of all mice assayed, however, HAR C57L/J males had the lowest levels of anti-BSA on both days. When strains differing in sensitivity to androgen and H-2 type were assayed for antibody to bovine gamma-globulin (B-IgG), RF/J females produced comparatively high antibody responses as expected for H-2k strains, but HAR RF/J males produced the lowest levels of antibody of all mice assayed.
The results of these studies support and extend earlier observations of an apparent correlation in male mice between high sensitivity to androgen and poor immune responsiveness.
Full text
PDF

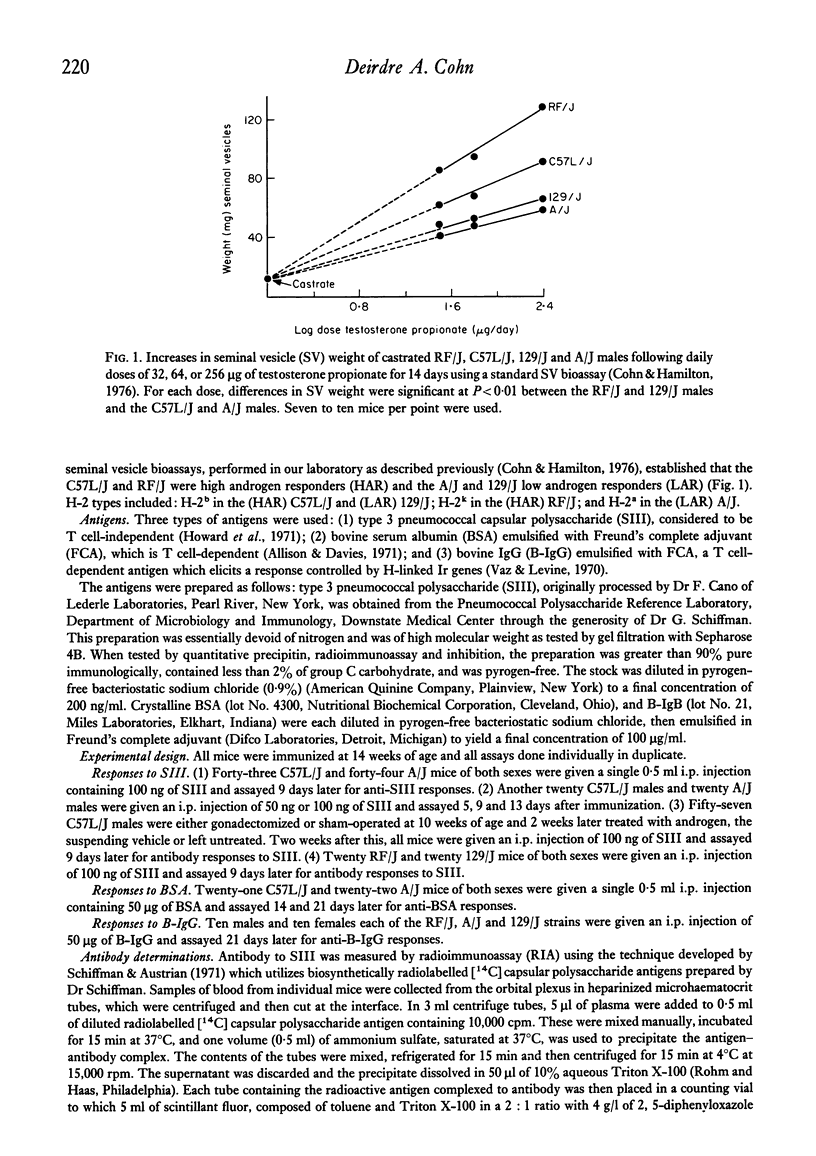


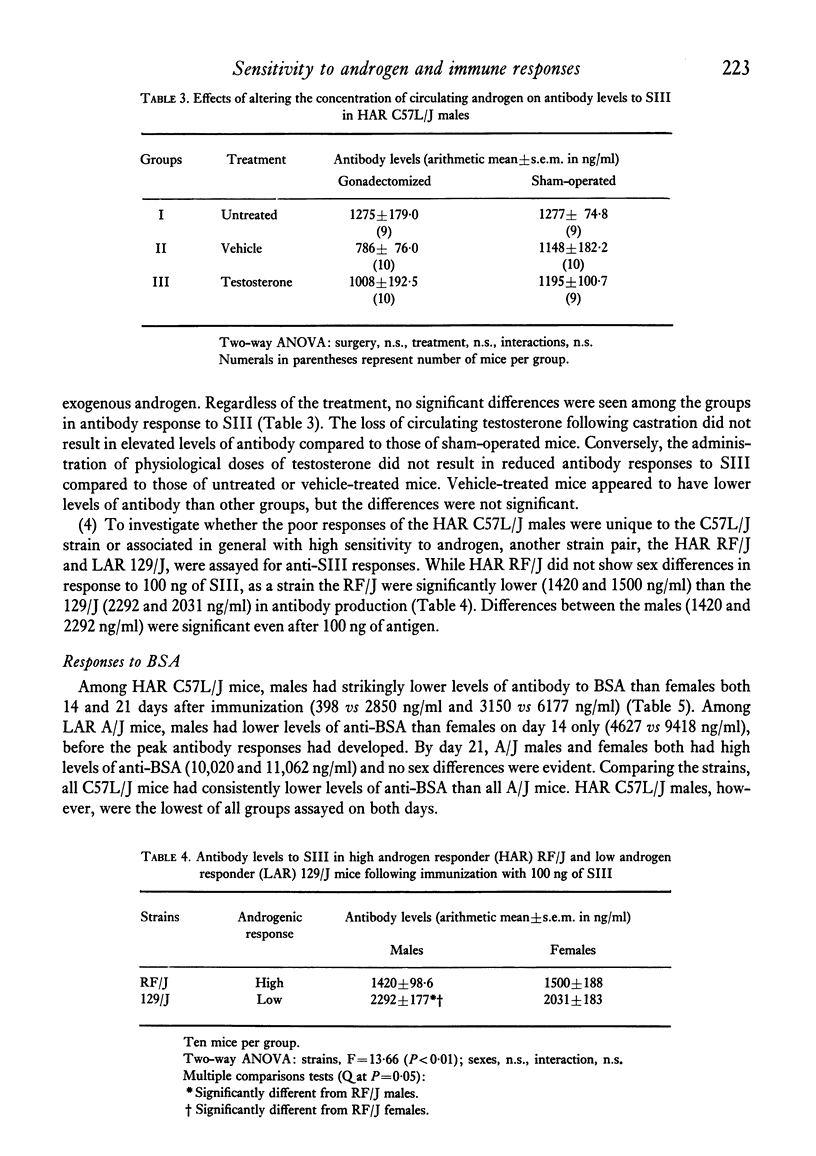
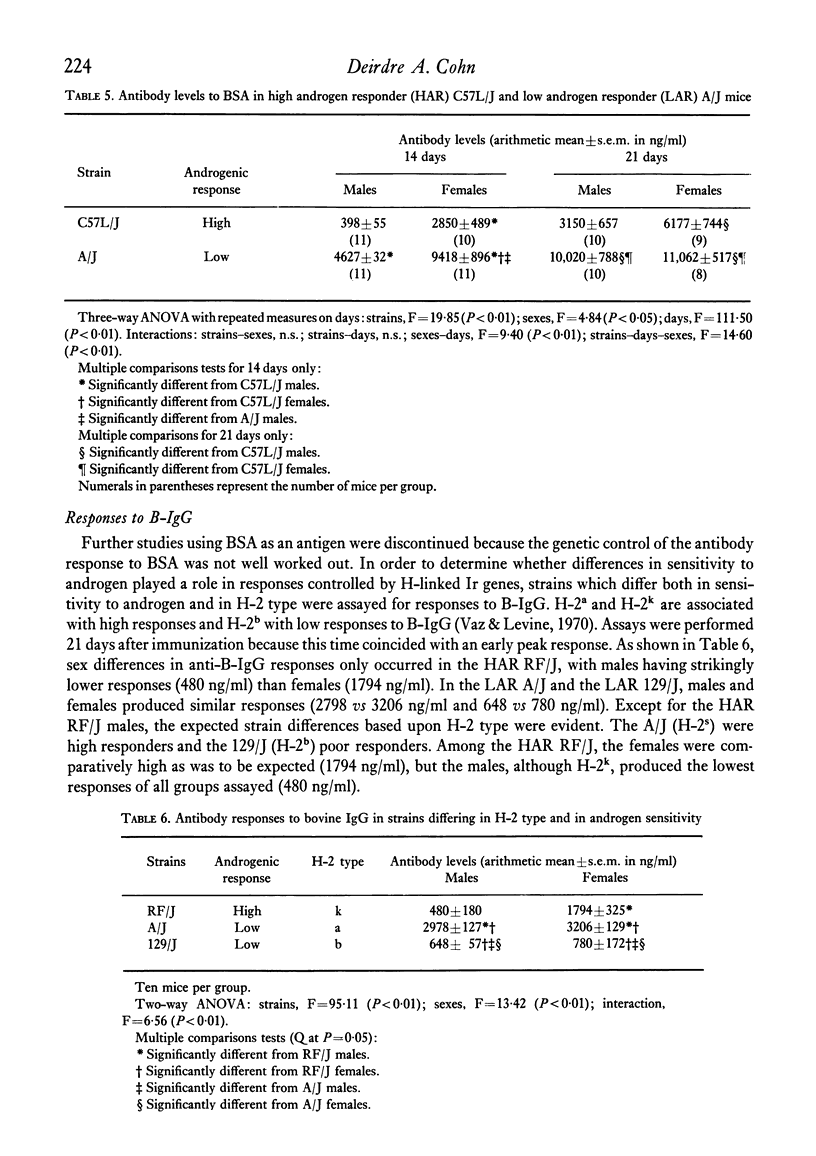



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovich D. R., Rowe P. Foetal plasma testosterone levels at mid-pregnancy and at term: relationship to foetal sex. J Endocrinol. 1973 Mar;56(3):621–622. doi: 10.1677/joe.0.0560621. [DOI] [PubMed] [Google Scholar]
- Adinolfi M., Haddad S. A., Seller M. J. X chromosome complement and serum levels of IgM in man and mouse. J Immunogenet. 1978 Jun;5(3):149–156. doi: 10.1111/j.1744-313x.1978.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Davies A. J. Requirement of thymus-dependent lymphocytes for potentiation by adjuvants of antibody formation. Nature. 1971 Oct 1;233(5318):330–332. doi: 10.1038/233330a0. [DOI] [PubMed] [Google Scholar]
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Asofsky R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. II. Relationship between IgM immunoglobulin levels and the ability to give an IgM antibody response. J Exp Med. 1974 Jun 1;139(6):1499–1512. doi: 10.1084/jem.139.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A. A., Hanson R. P. Influence of sex and age on natural resistance to St. Louis encephalitis virus infection in mice. Infect Immun. 1974 Jun;9(6):1123–1125. doi: 10.1128/iai.9.6.1123-1125.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Prescott B., Stashak P. W. Genetic control of the antibody response to type III pneumococcal polysaccharide in mice. III. Analysis of genes governing the expression of regulatory T cell activity. J Immunogenet. 1976 Aug;3(4):275–286. doi: 10.1111/j.1744-313x.1976.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Bartke A. Increased sensitivity of seminal vesicles to testosterone in a mouse strain with low plasma testosterone levels. J Endocrinol. 1974 Jan;60(1):145–148. doi: 10.1677/joe.0.0600145. [DOI] [PubMed] [Google Scholar]
- Bulmer R., Hancock K. W. Depletion of circulating T lymphocytes in pregnancy. Clin Exp Immunol. 1977 May;28(2):302–305. [PMC free article] [PubMed] [Google Scholar]
- Byron J. W. Effect of steroids on the cycling of haemopoietic stem cells. Nature. 1970 Dec 19;228(5277):1204–1204. doi: 10.1038/2281204a0. [DOI] [PubMed] [Google Scholar]
- Castro J. E. Orchidectomy and the immune response. II. Response of orchidectomized mice to antigens. Proc R Soc Lond B Biol Sci. 1974 Feb 26;185(1081):437–451. doi: 10.1098/rspb.1974.0028. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Garrett T. J. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972 Nov 1;136(5):1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- FEKETE E. A morphological study of the ovaries of virgin mice of eight inbred strains showing quantitative differences in their hormone producing components. Anat Rec. 1953 Sep;117(1):93–113. doi: 10.1002/ar.1091170108. [DOI] [PubMed] [Google Scholar]
- Friedman S. B., Grota L. J., Glasgow L. A. Differential susceptibility of male and female mice to encephalomyocarditis virus: effects of castration, adrenalectomy, and the administration of sex hormones. Infect Immun. 1972 May;5(5):637–644. doi: 10.1128/iai.5.5.637-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundbacher F. J. Human X chromosome carries quantitative genes for immunoglobulin M. Science. 1972 Apr 21;176(4032):311–312. doi: 10.1126/science.176.4032.311. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hamilton J. B., Hamilton R. S., Mestler G. E. Duration of life and causes of death in domestic cats: influence of sex, gonadectomy, and inbreeding. J Gerontol. 1969 Oct;24(4):427–437. doi: 10.1093/geronj/24.4.427. [DOI] [PubMed] [Google Scholar]
- Iványi P., Gregorová S., Micková M., Hampl R., Stárka L. Genetic association between a histocompatibility gene (H-2) and androgen metabolism in mice. Transplant Proc. 1973 Mar;5(1):189–191. [PubMed] [Google Scholar]
- Karayalcin G., Rosner F., Sawitsky A. Quantitative serum immunoglobulins in healthy Negroes. N Y State J Med. 1973 Mar 15;73(6):751–753. [PubMed] [Google Scholar]
- Lichtman M. A., Vaughan J. H., Hames C. G. The distribution of serum immunoglobulins, anti-gamma-G globulins ("rheumatoid factors") and antinuclear antibodies in White and Negro subjects in Evans County, Georgia. Arthritis Rheum. 1967 Jun;10(3):204–215. doi: 10.1002/art.1780100306. [DOI] [PubMed] [Google Scholar]
- MEYER R. K., RAO M. A., ASPINALL R. L. Inhibition of the development of the bursa of Fabricius in the embryos of the common fowl by 19-nortestosterone. Endocrinology. 1959 Jun;64(6):890–897. doi: 10.1210/endo-64-6-890. [DOI] [PubMed] [Google Scholar]
- Mathur S., Mathur R. S., Dowda H., Williamson H. O., Faulk W. P., Fudenberg H. H. Sex steroid hormones and antibodies to Candida albicans. Clin Exp Immunol. 1978 Jul;33(1):79–87. [PMC free article] [PubMed] [Google Scholar]
- Rhodes K., Markham R. L., Maxwell P. M., Monk-Jones M. E. Immunoglobulins and the X-chromosome. Br Med J. 1969 Aug 23;3(5668):439–441. doi: 10.1136/bmj.3.5668.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehm E. R., Fudenberg H. H. Serum levels of immune globulins in health and disease: a survey. Pediatrics. 1966 May;37(5):715–727. [PubMed] [Google Scholar]
- Strelkauskas A. J., Davies I. J., Dray S. Longitudinal studies showing alterations in the levels and functional response of T and B lymphocytes in human pregnancy. Clin Exp Immunol. 1978 Jun;32(3):531–539. [PMC free article] [PubMed] [Google Scholar]
- Terres G., Morrison S. L., Habicht G. S. A quantitative difference in the immune response between male and female mice. Proc Soc Exp Biol Med. 1968 Mar;127(3):664–667. doi: 10.3181/00379727-127-32768. [DOI] [PubMed] [Google Scholar]
- Vaz N. M., Levine B. B. Immune responses of inbred mice to repeated low doses of antigen: relationship to histocompatibility (H-2) type. Science. 1970 May 15;168(3933):852–854. doi: 10.1126/science.168.3933.852. [DOI] [PubMed] [Google Scholar]
- WARNER N. L., SZENBERG A., BURNET F. M. The immunological role of different lymphoid organs in the chicken. I. Dissociation of immunological responsiveness. Aust J Exp Biol Med Sci. 1962 Oct;40:373–387. doi: 10.1038/icb.1962.42. [DOI] [PubMed] [Google Scholar]
- WASHBURN T. C., MEDEARIS D. N., Jr, CHILDS B. SEX DIFFERENCES IN SUSCEPTIBILITY TO INFECTIONS. Pediatrics. 1965 Jan;35:57–64. [PubMed] [Google Scholar]
- Wood C. B., Martin W., Adinolfi M., Polani P. E. Immunoglobulins and the X-chromosome. Br Med J. 1969 Oct 11;4(5675):110–110. doi: 10.1136/bmj.4.5675.110-a. [DOI] [PMC free article] [PubMed] [Google Scholar]


