Abstract
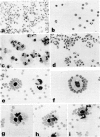
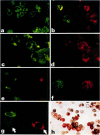
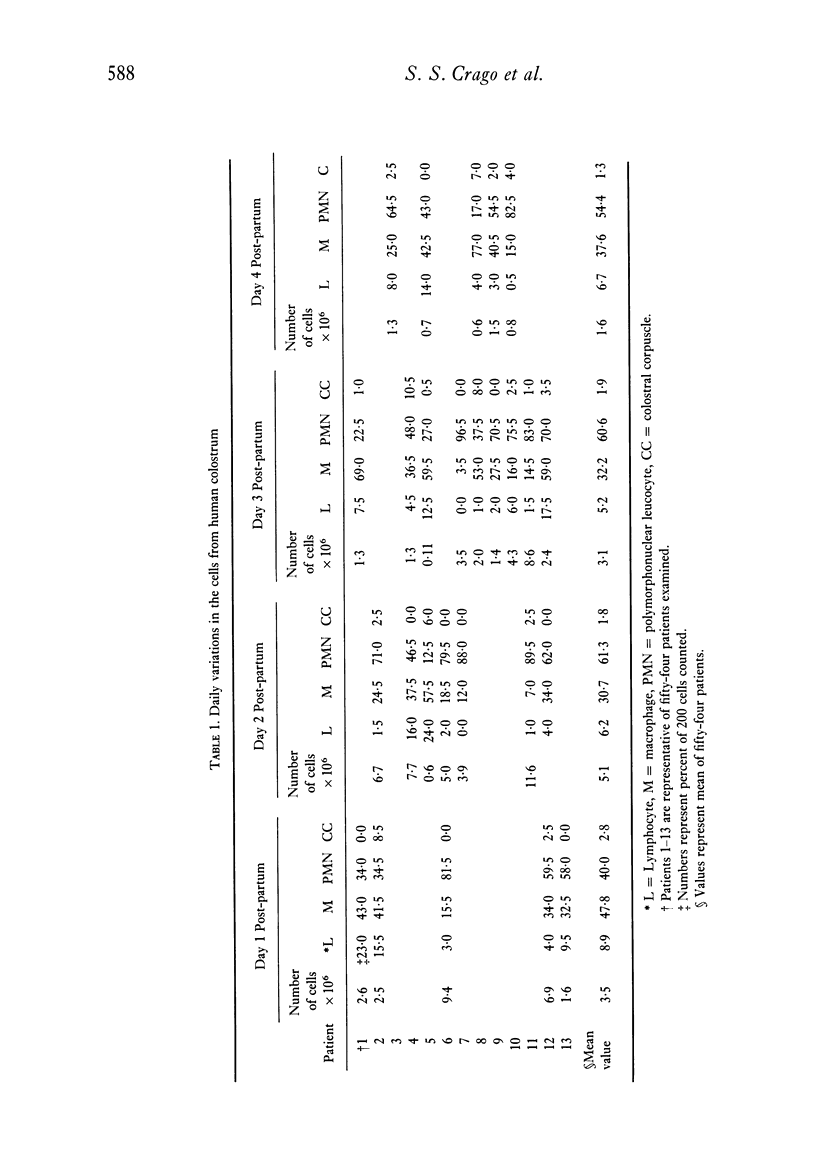
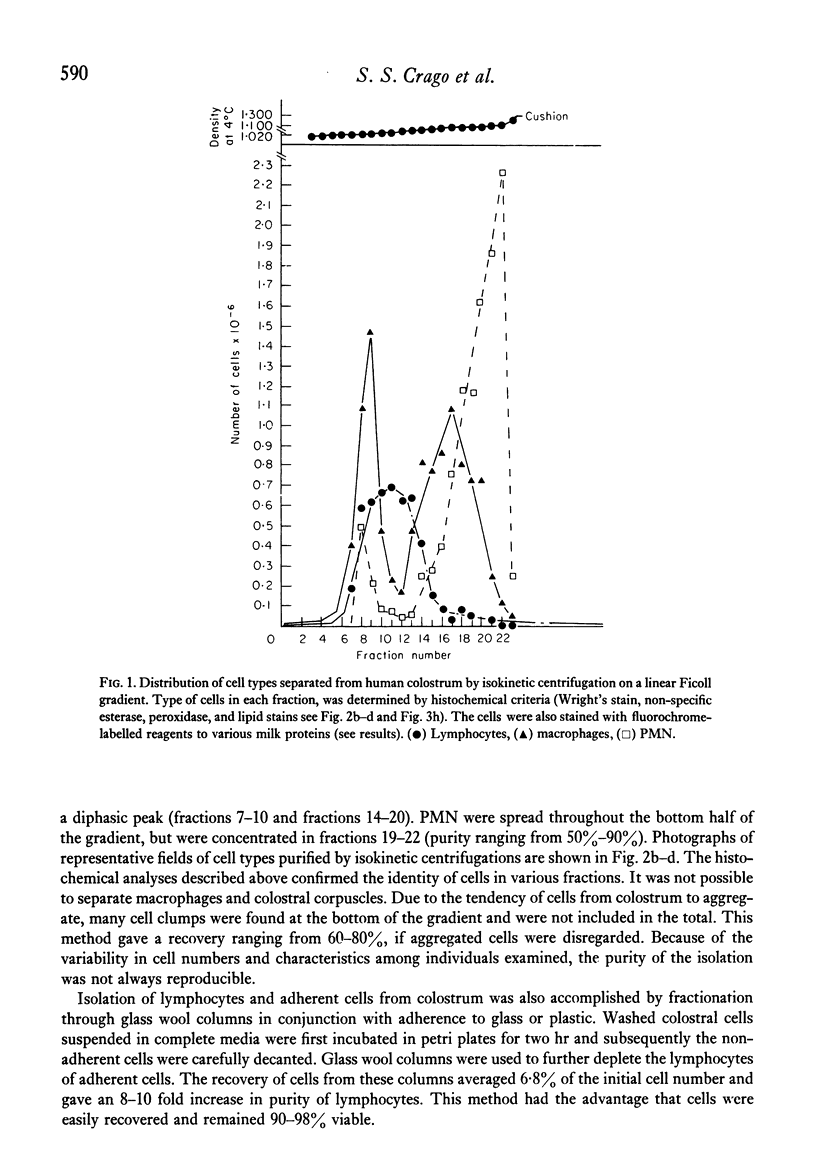
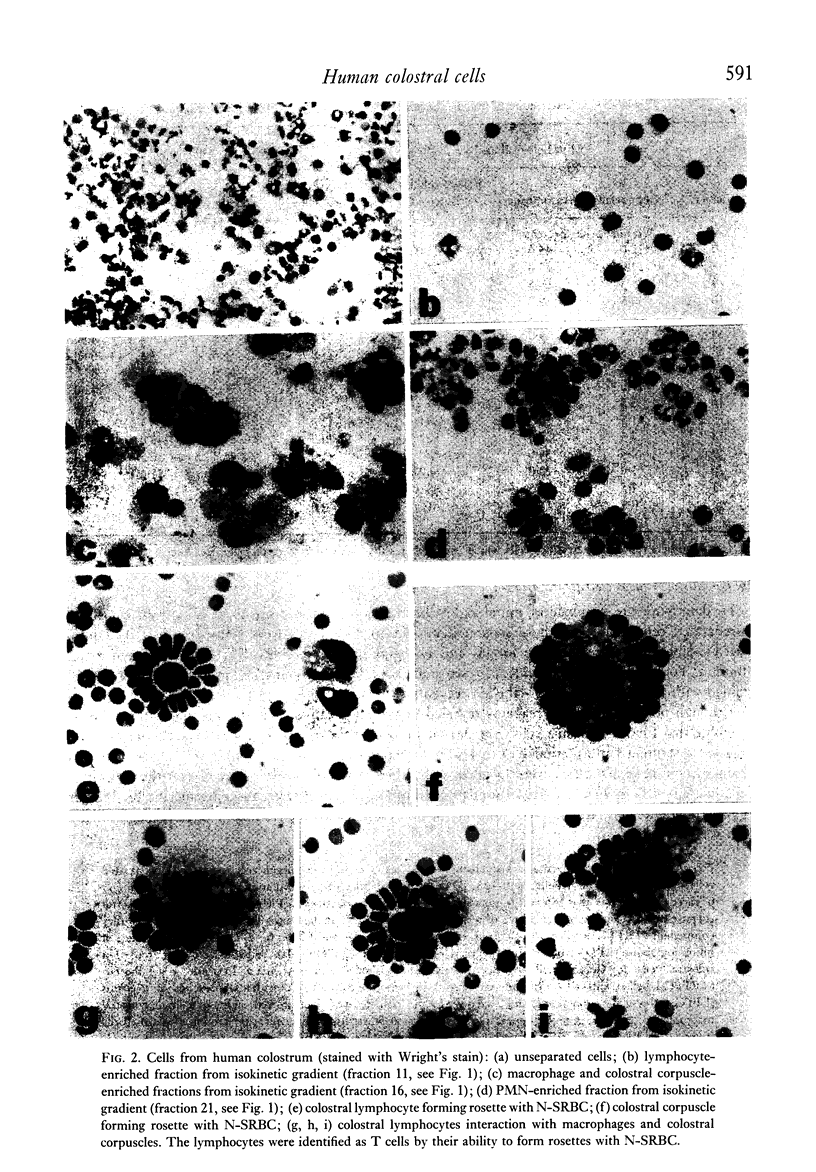
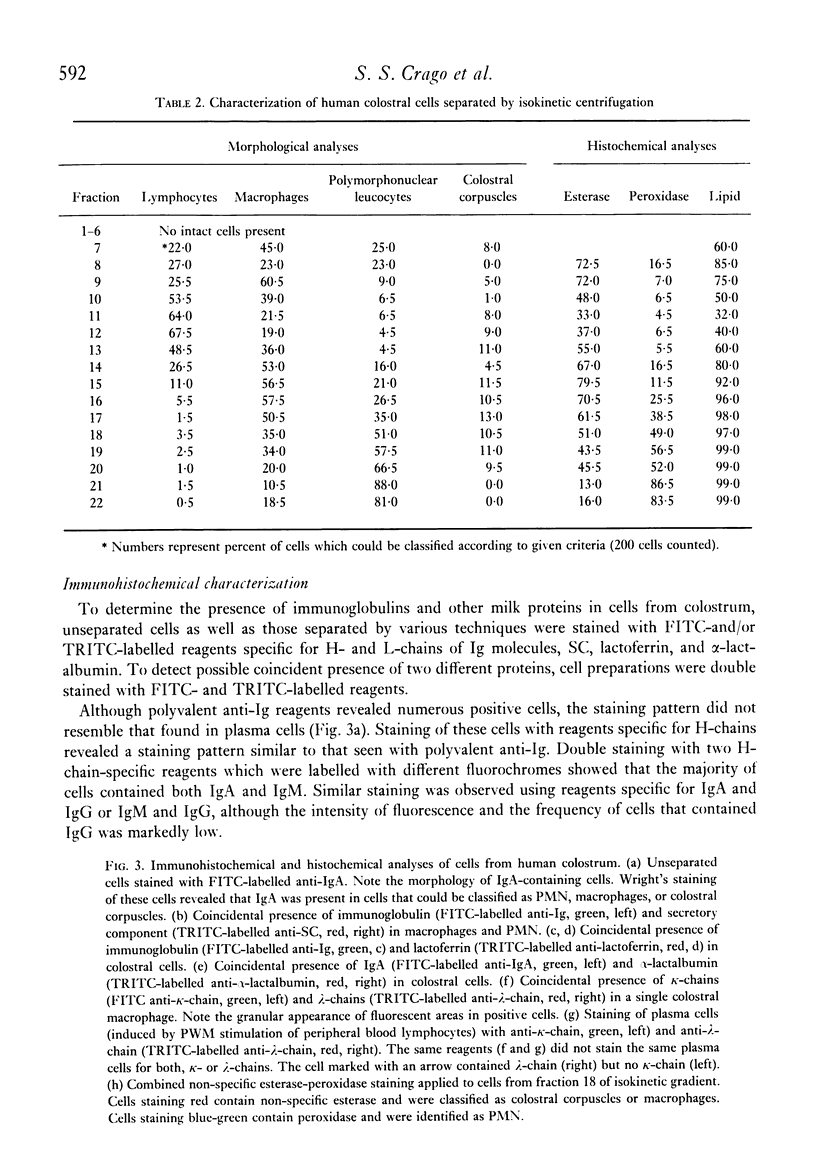
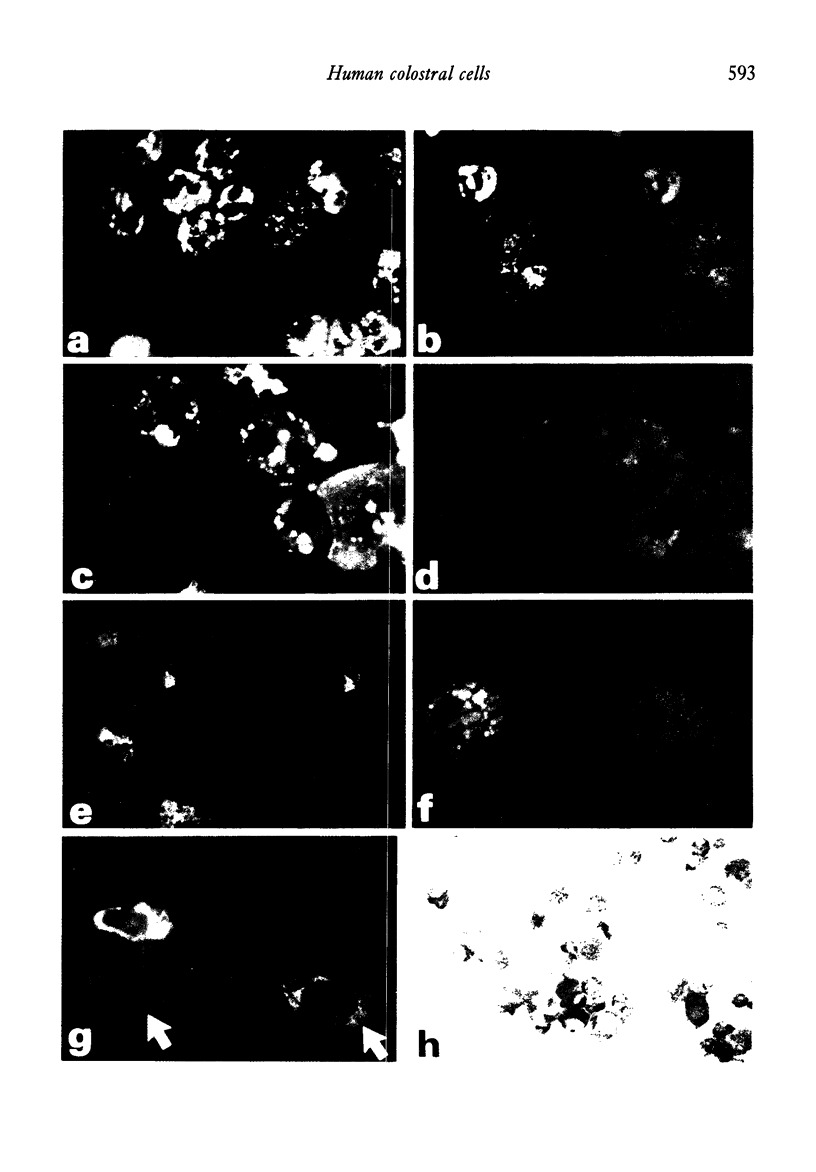
Analyses of the cells present in human colostrum obtained from fifty-four healthy donors during the first four days of lactation revealed that there were 3.3 x 10(6) (range 1.1 x 10(5)--1.2 x 10(7)) cells per ml of colostrum. Based on histochemical examinations, it was found that this population consisted of 30--47% macrophages, 40--60% polymorphonuclear leucocytes, 5.2--8.9% lymphocytes, and 1.3--2.8% colostral corpuscles; epithelial cells were rarely encountered. The identity of various cell types was confirmed by Wright's stain and by a series of histochemical techniques which disclosed the presence of non-specific esterase, peroxidase, and lipids. For further characterization, the different types of cells were separated by various methods, such as Ficoll-Hypaque density centrifugation, isokinetic centrifugation on a linear Ficoll gradient, adherence to glass or plastic, and phagocytosis of carbonyl iron. Immunohistochemical staining with FITC- and/or TRITC-labelled reagents to IgA, IgM, IgG, K- and lambda-chains, secretory component, lactoferrin, and alpha-lactalbumin were applied to unseparated as well as separated colostral cells. Polymorphonuclear leucocytes (staining for peroxidase) as well as macrophages and colostral corpuscles (staining for non-specific esterase) exhibited numerous intracellular vesicles that contained lipids as well as various combinations of milk proteins. Lymphoid cells did not stain with any of these reagents and plasma cells were not detected among the colostral cells. Individual phagocytic cells contained immunoglobulins of the IgA and IgM classes, both K and lambda light chains, secretory component, lactoferrin, and alpha-lactalbumin. The coincidental appearance of these proteins in single, phagocytic cells but not in lymphoid cells indicate that the cells acquired these proteins by ingestion from the environment. Markers commonly used for the identification of B lymphocytes (surface immunoglobulins) and T lymphocytes (receptors for sheep red blood cells) were unreliable for the analysis of colostral cells (unless accompanied by subsequent morphological characterization) because strong fluorescence was observed on the surface of many non-lymphoid cells and because numerous macrophages and colostral corpuscles formed rosettes with sheep red blood cells (SRBC). Lymphocytes, often found in association with colostral macrophages or corpuscles, were classified as T cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlstedt S., Carlsson B., Hanson L. A., Goldblum R. M. Antibody production by human colostral cells. I. Immunoglobulin class, specificity, and quantity. Scand J Immunol. 1975 Sep;4(5-6):535–539. doi: 10.1111/j.1365-3083.1975.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Mestecky J., McGhee J. R. Naturally occurring secretory immunoglobulin A antibodies to Streptococcus mutans in human colostrum and saliva. Infect Immun. 1976 Aug;14(2):355–362. doi: 10.1128/iai.14.2.355-362.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., McDermott M., Befus D., O'Neill M. A common mucosal immunologic system involving the bronchus, breast and bowel. Adv Exp Med Biol. 1978;107:53–59. doi: 10.1007/978-1-4684-3369-2_7. [DOI] [PubMed] [Google Scholar]
- Brodbeck U., Denton W. L., Tanahashi N., Ebner K. E. The isolation and identification of the B protein of lactose synthetase as alpha-lactalbumin. J Biol Chem. 1967 Apr 10;242(7):1391–1397. [PubMed] [Google Scholar]
- Carlsson B., Gothefors L., Ahlstedt S., Hanson L. A., Winberg J. Studies of Escherichia coli O antigen specific antibodies in human milk, maternal serum and cord blood. Acta Paediatr Scand. 1976 Mar;65(2):216–224. doi: 10.1111/j.1651-2227.1976.tb16541.x. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Gearhart P. J., Kamat R., Robertson S. M., Tseng J. Origin and differentiation of lymphocytes involved in the secretory IgA responses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):201–215. doi: 10.1101/sqb.1977.041.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago S. S., Mestecky J. Secretory component: interactions with intracellular and surface immunoglobulins of human lymphoid cells. J Immunol. 1979 Mar;122(3):906–911. [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Jouanen E., Williams R. C., Jr T and B lymphocytes in human colostrum. Clin Immunol Immunopathol. 1974 Nov;3(2):248–255. doi: 10.1016/0090-1229(74)90011-7. [DOI] [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Goldman A. S., Smith C. W. Host resistance factors in human milk. J Pediatr. 1973 Jun;82(6):1082–1090. doi: 10.1016/s0022-3476(73)80453-6. [DOI] [PubMed] [Google Scholar]
- Hanson L. A., Ahlstedt S., Carlsson B., Kaijser B., Larsson P., Baltzer I. M., Akerlund A. S., Edén C. S., Svennerholm A. M. Secretory IgA antibodies to enterobacterial virulence antigens: their induction and possible relevance. Adv Exp Med Biol. 1978;107:165–176. doi: 10.1007/978-1-4684-3369-2_20. [DOI] [PubMed] [Google Scholar]
- Hanson L. A., Winberg J. Breast milk and defence against infection in the newborn. Arch Dis Child. 1972 Dec;47(256):845–848. doi: 10.1136/adc.47.256.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles A. K., Gurner B. W., Coombs R. R. Some properties of human colostral cells. Aust J Exp Biol Med Sci. 1969 Jun;47(3):349–360. doi: 10.1038/icb.1969.38. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Farrar J. J., Michalek S. M., Mergenhagen S. E., Rosenstreich D. L. Cellular requirements for lipopolysaccharide adjuvanticity. A role for both T lymphocytes and macrophages for in vitro responses to particulate antigens. J Exp Med. 1979 Apr 1;149(4):793–807. doi: 10.1084/jem.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Arnold R. R., Michalek S. M., Prince S. J., Babb J. L. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J Clin Invest. 1978 Mar;61(3):731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Michalek S. M., Arnold R. R., Crago S. S., Babb J. L. Concept of the local and common mucosal immune response. Adv Exp Med Biol. 1978;107:185–192. doi: 10.1007/978-1-4684-3369-2_22. [DOI] [PubMed] [Google Scholar]
- Mohr J. A. The possible induction and-or acquisition of cellular hypersensitivity associated with ingestion of colostrum. J Pediatr. 1973 Jun;82(6):1062–1064. doi: 10.1016/s0022-3476(73)80448-2. [DOI] [PubMed] [Google Scholar]
- Murillo G. J., Goldman A. S. The cells of human colostrum. II. Synthesis of IgA and Beta1c. Pediatr Res. 1970 Jan;4(1):71–75. doi: 10.1203/00006450-197001000-00008. [DOI] [PubMed] [Google Scholar]
- Ogra S. S., Weintraub D. I., Ogra P. L. Immunologic aspects of human colostrum and milk: interaction with the intestinal immunity of the neonate. Adv Exp Med Biol. 1978;107:95–107. doi: 10.1007/978-1-4684-3369-2_12. [DOI] [PubMed] [Google Scholar]
- Ogra S. S., Weintraub D., Ogra P. L. Immunologic aspects of human colostrum and milk. III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J Immunol. 1977 Jul;119(1):245–248. [PubMed] [Google Scholar]
- Parmely M. J., Beer A. E., Billingham R. E. In vitro studies on the T-lymphocyte population of human milk. J Exp Med. 1976 Aug 1;144(2):358–370. doi: 10.1084/jem.144.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmely M. J., Beer A. E. Colostral cell-mediated immunity and the concept of a common secretory immune system. J Dairy Sci. 1977 Apr;60(4):655–665. doi: 10.3168/jds.S0022-0302(77)83915-5. [DOI] [PubMed] [Google Scholar]
- Parmely M. J., Reath D. B., Beer A. E., Billingham R. E. Cellular immune responses of human milk T lymphocytes to certain environmental antigens. Transplant Proc. 1977 Jun;9(2):1477–1483. [PubMed] [Google Scholar]
- Pittard W. B., 3rd, Polmar S. H., Fanaroff A. A. The breastmilk macrophage: a potential vehicle for immunoglobulin transport. J Reticuloendothel Soc. 1977 Dec;22(6):597–603. [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, weir E. E., Zettergren J. G. Problems connected with the separation of different kinds of cells. Int Rev Exp Pathol. 1975;14:91–204. [PubMed] [Google Scholar]
- Pretlow T. G. Estimation of experimental conditions that permit cell separations by velocity sedimentation on isokinetic gradients of Ficoll in tissue culture medium. Anal Biochem. 1971 May;41(1):248–255. doi: 10.1016/0003-2697(71)90207-7. [DOI] [PubMed] [Google Scholar]
- Roux M. E., McWilliams M., Phillips-Quagliata J. M., Weisz-Carrington P., Lamm M. E. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977 Nov 1;146(5):1311–1322. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Schlesinger J. J., Covelli H. D. Evidence for transmission of lymphocyte responses to tuberculin by breast-feeding. Lancet. 1977 Sep 10;2(8037):529–532. doi: 10.1016/s0140-6736(77)90665-1. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Goldman A. S. Interactions of lymphocytes and macrophages from human colostrum: characteristics of the interacting lymphocyte. J Reticuloendothel Soc. 1970 Jul;8(1):91–104. [PubMed] [Google Scholar]
- Smith C. W., Goldman A. S. The cells of human colostrum. I. In vitro studies of morphology and functions. Pediatr Res. 1968 Mar;2(2):103–109. doi: 10.1203/00006450-196803000-00005. [DOI] [PubMed] [Google Scholar]
- Welsh J. K., May J. T. Anti-infective properties of breast milk. J Pediatr. 1979 Jan;94(1):1–9. doi: 10.1016/s0022-3476(79)80340-6. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]