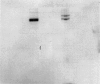
Abstract
Metabolic studies were performed with a purified, functionally-active preparation of human beta 1H. In seven normal human subjects, the half-life ranged from 66--87 hr with fractional catabolic rates (FCR) of 1.04--1.63%/hr. Synthesis rates were 0.22--0.57 mg/kg/hr and extravascular distribution ratios were 0.34--0.67. There was evidence of extra-vascular catabolism in each subject. In sixteen patients with immunological disease four showed hypercatabolism of beta 1H. However, three patients with C3 mephritis factor (NeF) had normal beta 1H turnover despite profound reduction in C3 concentration; it is suggested that the reaction of beta 1H with the C3b. Bb convertase exposes it to a catabolic site and that in the NeF patients the NeF stabilized convertase prevents such exposure. Studies of the acute phase response were carried out in nine patients following elective surgery, with C-reactive protein (CRP) used as the control protein: six patients showed no rise in beta 1H levels and three showed a small (20%) rise whereas all exhibited a gross rise in CRP. Pre-incubation of 125I- beta 1H with NHS, with NHS in the presence of NeF and with C3b+C3b 1NA caused no change in beta 1H turnover in animals despite demonstrable total C3 conversion with the NeF.
Full text
PDF

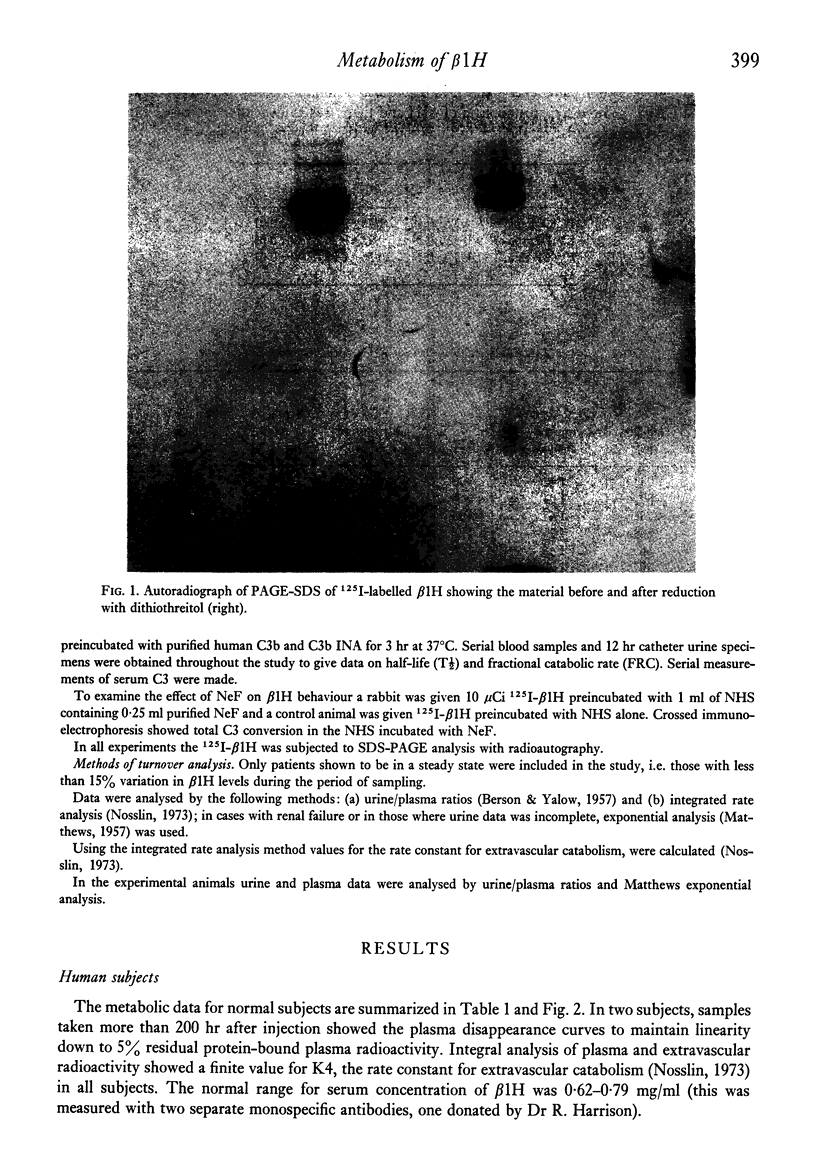
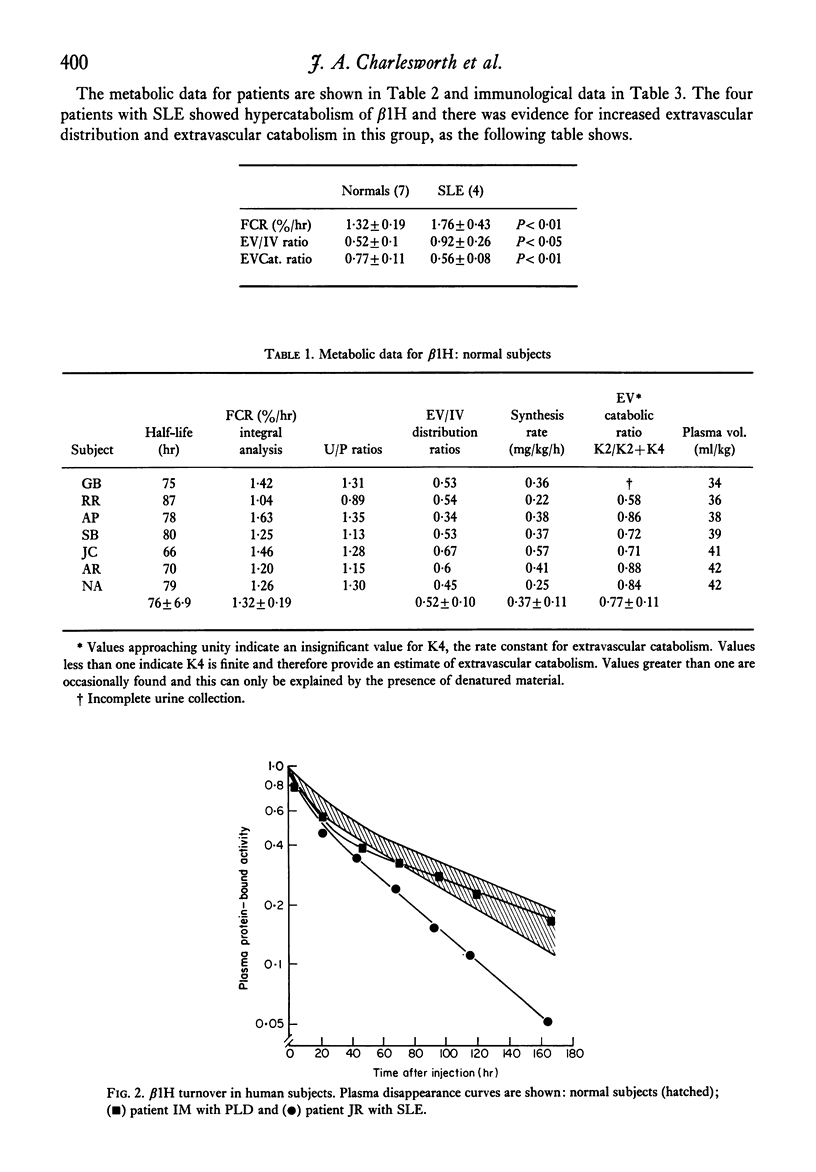
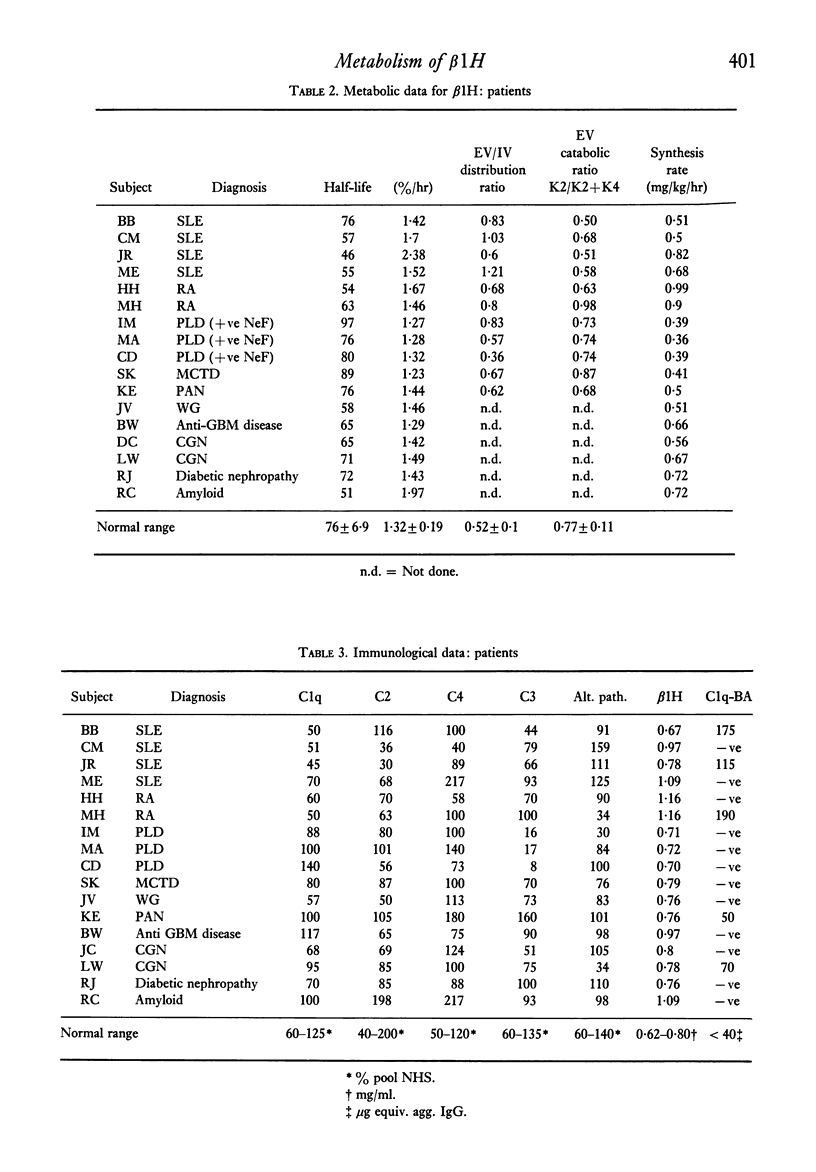



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos N., Sissons J. G., Girard J. F., Lachmann P. J., Peters D. K. The cofactors required by C3 nephritic factor to generate a C3 convertase in vitro. Clin Exp Immunol. 1976 Jun;24(3):474–482. [PMC free article] [PubMed] [Google Scholar]
- Charlesworth J. A., Williams D. G., Sherington E., Lachmann P. J., Peters D. K. Metabolic studies of the third component of complement and the glycine-rich beta glycoprotein in patients with hypocomplementemia. J Clin Invest. 1974 Jun;53(6):1578–1587. doi: 10.1172/JCI107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth J. A., Williams D. G., Sherington E., Peters D. K. Metabolism of the third component of complement (C3) in normal human subjects. Clin Sci Mol Med. 1974 Feb;46(2):223–229. doi: 10.1042/cs0460223. [DOI] [PubMed] [Google Scholar]
- Conrad D. H., Carlo J. R., Ruddy S. Interaction of beta1H globulin with cell-bound C3b: quantitative analysis of binding and influence of alternative pathway components on binding. J Exp Med. 1978 Jun 1;147(6):1792–1805. doi: 10.1084/jem.147.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daha M. R., Fearon D. T., Austen K. F. C3 nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol. 1976 Jan;116(1):1–7. [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975 Oct 1;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Martin A., Lachmann P. J., Halbwachs L., Hobart M. J. Haemolytic diffusion plate assays for factors B and D of the alternative pathway of complement activation. Immunochemistry. 1976 Apr;13(4):317–324. doi: 10.1016/0019-2791(76)90341-4. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Nosslin B. Analysis of disappearance time-curves after single injection of labelled proteins. Ciba Found Symp. 1972;9:113–130. doi: 10.1002/9780470719923.ch7. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Markham R. E., Thomas H. C., Williams B. D., Petrie A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol. 1978 Apr;32(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- Peters D. K., Martin A., Weinstein A., Cameron J. S., Barratt T. M., Ogg C. S., Lachmann P. J. Complement studies in membrano-proliferative glomerulonephritis. Clin Exp Immunol. 1972 Jul;11(3):311–320. [PMC free article] [PubMed] [Google Scholar]
- Pussell B. A., Lockwood C. M., Scott D. M., Pinching A. J., Peters D. K. Value of immune-complex assays in diagnosis and management. Lancet. 1978 Aug 12;2(8085):359–364. doi: 10.1016/s0140-6736(78)92954-9. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J Immunol. 1971 Sep;107(3):742–750. [PubMed] [Google Scholar]
- Sissons J. G., Liebowitch J., Amos N., Peters D. K. Metabolism of the fifth component of complement, and its relation to metabolism of the third component, in patients with complement activation. J Clin Invest. 1977 Apr;59(4):704–715. doi: 10.1172/JCI108689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]