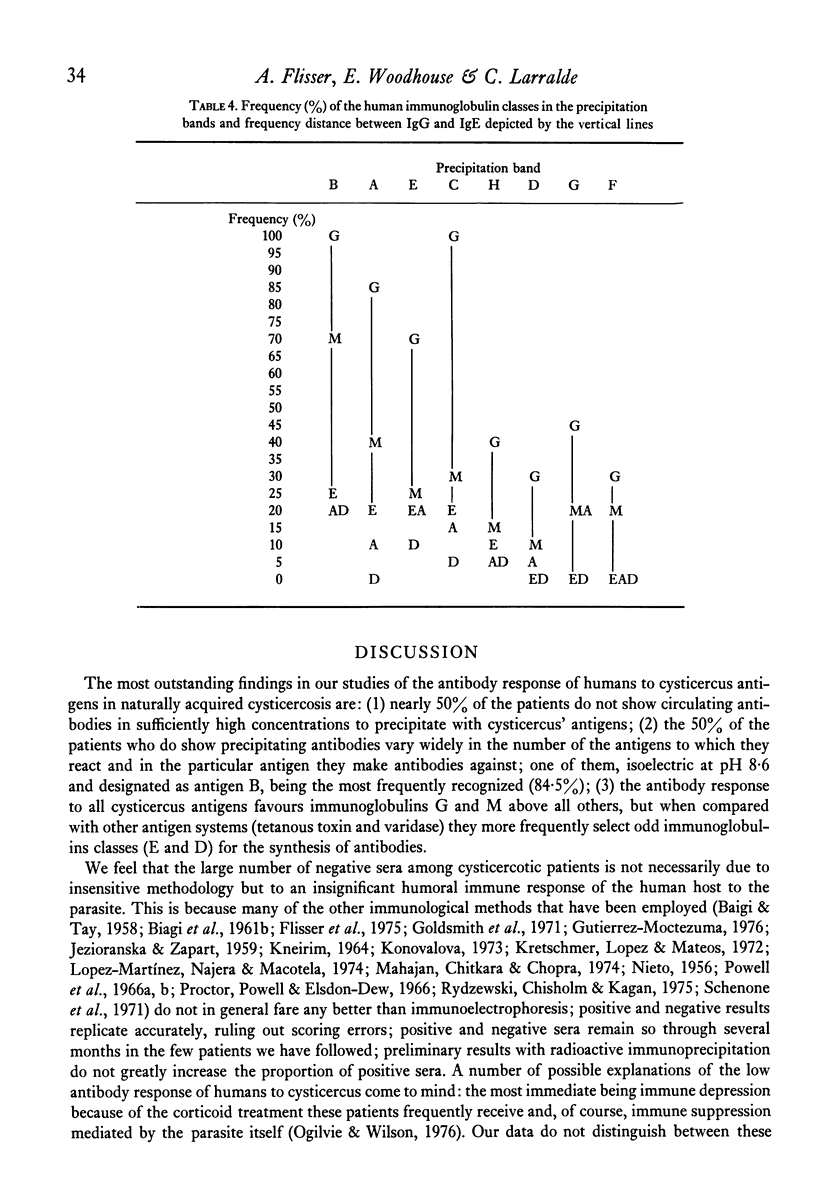
Abstract
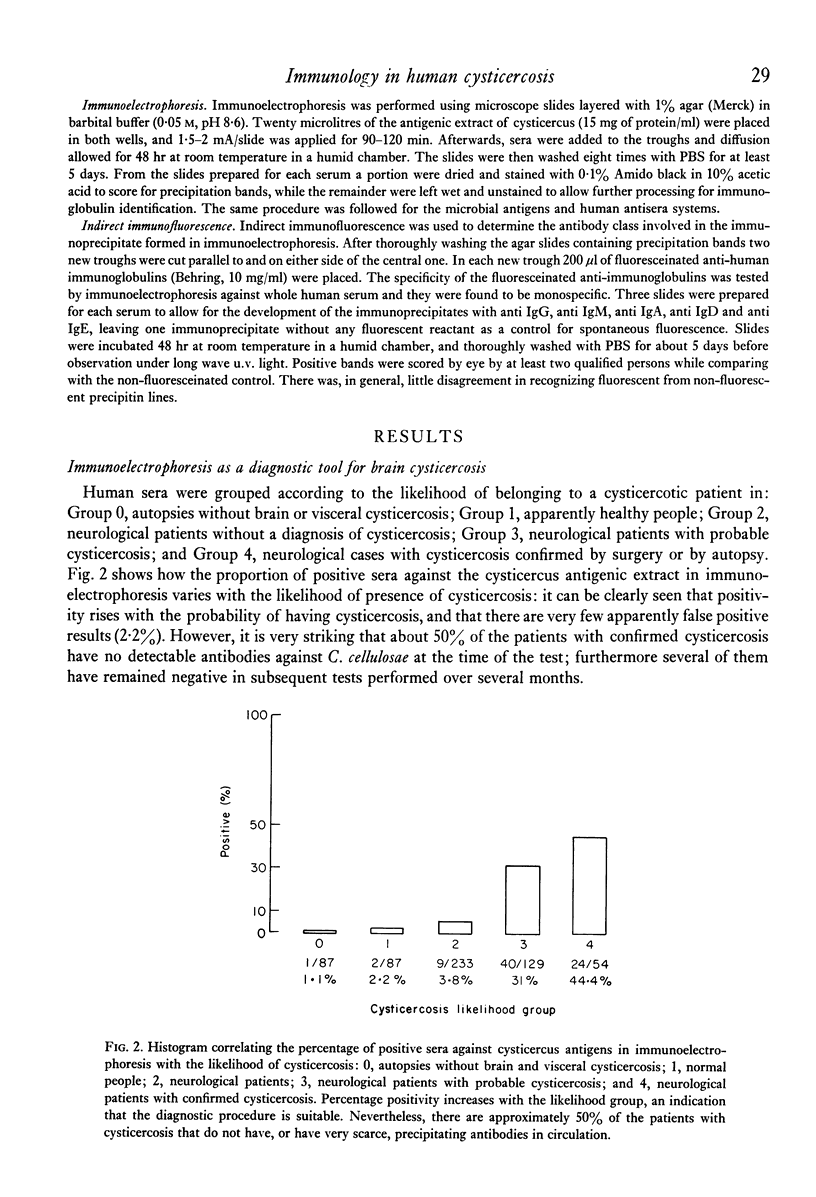
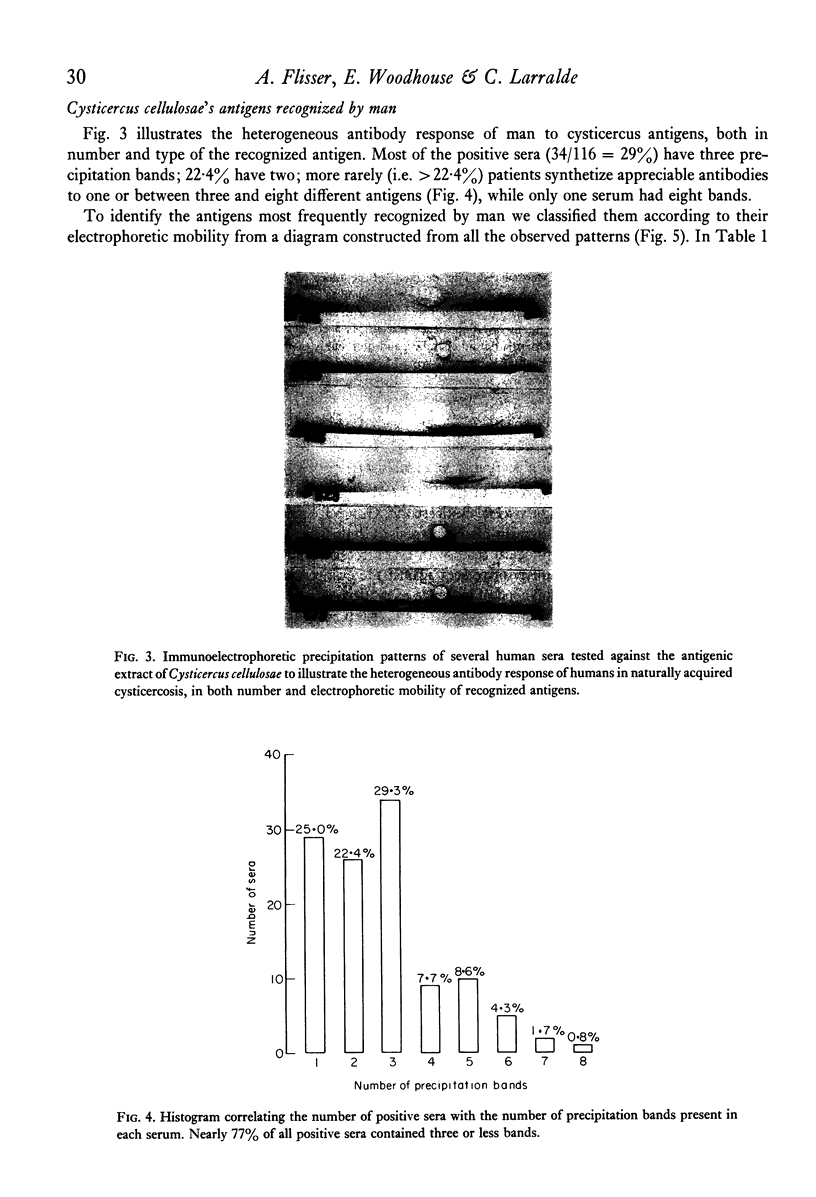
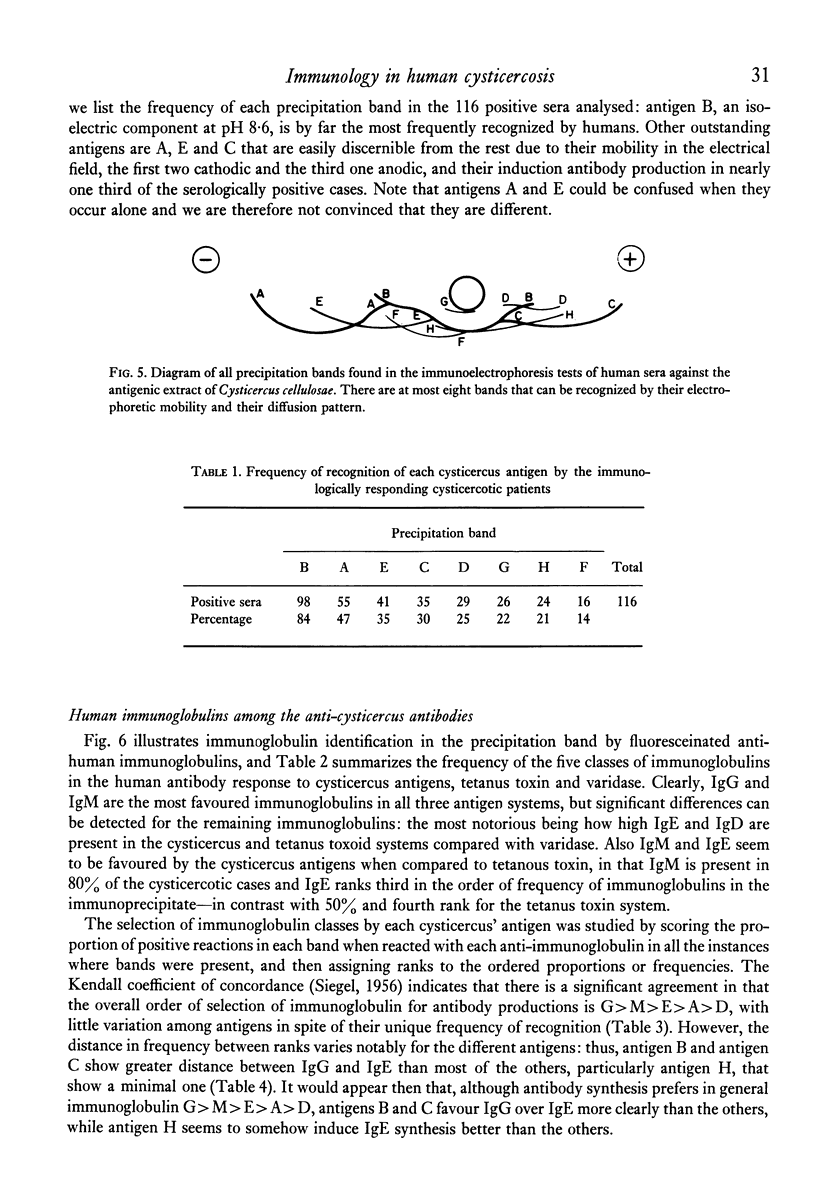
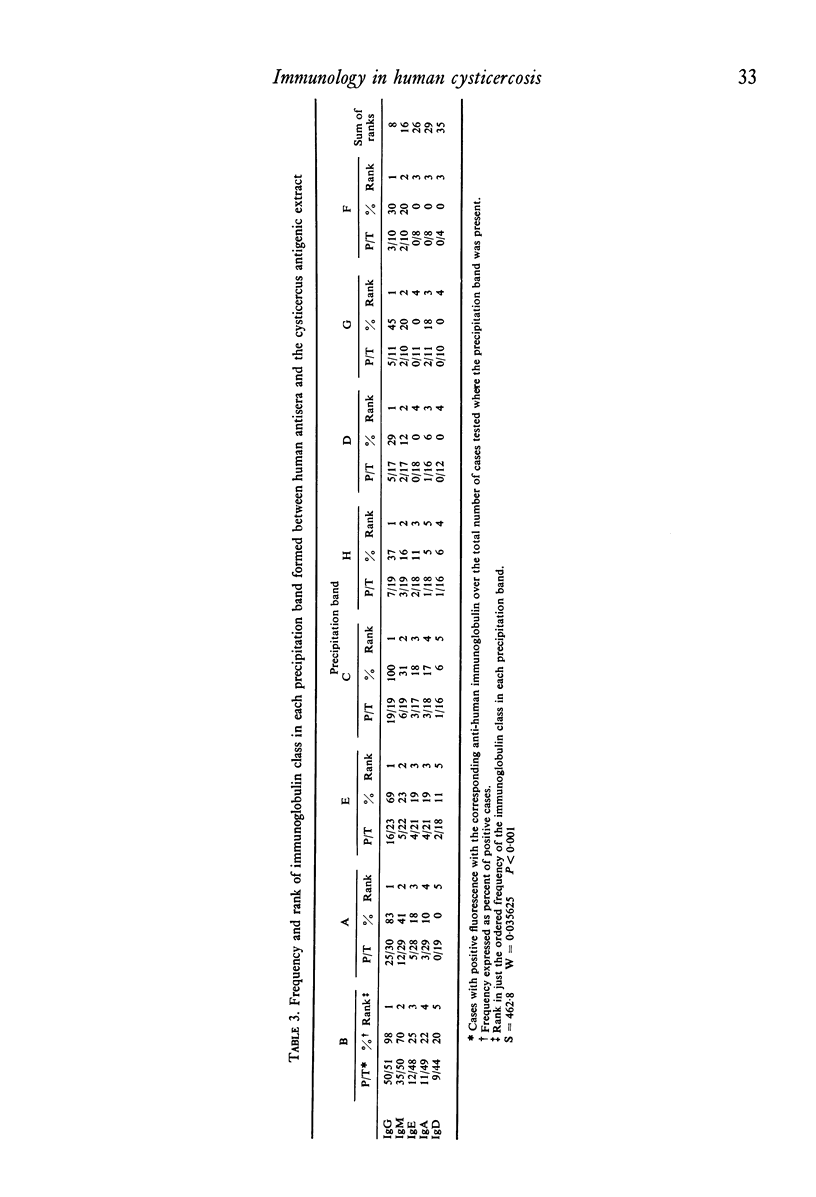
Immunoelectrophoresis of sera from patients with brain cysticercosis against a crude antigenic extract from Cysticercus cellulosae indicates that nearly 50% of the patients do not make sufficient antibodies to ostensively precipitate. The other 50% of the patients who do make precipitating antibodies show a very heterogeneous response in the number of antigens they recognize as well as in the type of antigen--as classified by their electrophoretic mobilities. The most favoured, called antigen B, is recognized by 84% of positive sera and corresponds to one or a limited number of antigens isoelectric at pH 8.6. Indirect immunofluorescence with monospecific anti-human immunoglobulins, performed upon the immunoelectrophoretic preparations, reveal that all cysticercus antigens induced the synthesis of antibodies in the immunoglobulin classes in the order G greater than M greater than E greater than A greater than D. Finally, antigen H (an anodic component) seems to favour IgE relative to its ability to induce IgG. Thus, although in natural infection a good proportion of cysticercotic patients do not seem to mount an energetic antibody response against the parasite, giving rise to some speculations about immunosuppression, the fact that 50% do synthesize antibodies allows for some optimistic expectations from vaccination of humans--in view of the good results of vaccination in experimental animals mediated by IgG antibodies. A likely prospect for a human vaccine would be antigen B because it is the most frequently detected by humans, although its immunizing and toxic properties remain to be properly studied.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIAGI F., TAY J. A precipitation reaction for the diagnosis of cysticercosis. Am J Trop Med Hyg. 1958 Jan;7(1):63–65. doi: 10.4269/ajtmh.1958.7.63. [DOI] [PubMed] [Google Scholar]
- Blundell S. K., Gemmell M. A., Macnamara F. N. Immunological responses of the mammalian host against tapeworm infections. VI. Demonstration of humoral immunity in sheep induced by the activated embryos of Taenia hydatigena and T. ovis. Exp Parasitol. 1968 Aug;23(1):79–82. doi: 10.1016/0014-4894(68)90044-1. [DOI] [PubMed] [Google Scholar]
- Dessaint J. P., Bout D., Wattre P., Capron A. Quantitative determination of specific IgE antibodies to Echinococcus granulosus and IgE levels in sera from patients with hydatid disease. Immunology. 1975 Nov;29(5):813–823. [PMC free article] [PubMed] [Google Scholar]
- Flisser A., Bulnes I., Diaz M. L., Luna R., Woodhouse E., Beltran F., Martinez I., Larralde C. Estudio seroepidemiológico de la cisticercósis humana en problaciones predominantemente indígenas y rurales del estado de Chiapas. Arch Invest Med (Mex) 1976;7(3):107–113. [PubMed] [Google Scholar]
- Goldsmith R. S., Kagan I. G., Reyes-González M. A., Cedeño Ferreira J. Estudios seroepidemiológicos realizodos en Oaxaca, Mexico. I. Encuesta de anticuerpos parasitarios mediante la prueba de hemaglutinacón indirecta. Bol Oficina Sanit Panam. 1971 Dec;71(6):500–518. [PubMed] [Google Scholar]
- Hogarth-Scott R. S., Johansson S. G., Bennich H. Antibodies to Toxocara in the sera of visceral larva migrans patients: the significance of raised levels of IgE. Clin Exp Immunol. 1969 Dec;5(6):619–625. [PMC free article] [PubMed] [Google Scholar]
- JEZIORANSKA A., ZAPART W. [Results of serological studies on cysticercosis]. Pol Tyg Lek. 1959 Jul 13;14:1281–1285. [PubMed] [Google Scholar]
- Kojima S., Yokogawa M., Tada T. Raised levels of serum IgE in human helminthiases. Am J Trop Med Hyg. 1972 Nov;21(6):913–918. doi: 10.4269/ajtmh.1972.21.913. [DOI] [PubMed] [Google Scholar]
- Kretschmer R., López-Osuna M., Humberto Mateos J. Nuevas perspectivas en el diagnóstico de la cisticercosis. Gac Med Mex. 1972 Mar;103(3):242–246. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leid R. W., Williams J. F. The immunological response of the rat to infection with Taenia taeniaeformis. I. Immunoglobulin classes involved in passive transfer of resistance. Immunology. 1974 Aug;27(2):195–208. [PMC free article] [PubMed] [Google Scholar]
- López Martińez R., Nájera Oliver O., Macotela Ruíz E. Cisticercoósis cutánea. Informe de cinco casos. Prensa Med Mex. 1974 Jan-Feb;39(1-2):1–4. [PubMed] [Google Scholar]
- Mahajan R. C., Chitkara N. L., Chopra J. S. Evaluation of cysticercous and adult worm antigens in serodiagnosis of cysticercosis. Indian J Med Res. 1974 Sep;62(9):1310–1313. [PubMed] [Google Scholar]
- Musoke A. J., Williams J. F. Immunoglobulins associated with passive transfer of resistance to Taenia taeniaeformis in the mouse. Immunology. 1975 Jan;28(1):97–101. [PMC free article] [PubMed] [Google Scholar]
- Musoke A. J., Williams J. F. Immunological response of the rat to infection with Taenia taeniaeformis: protective antibody response to implanted parasites. Int J Parasitol. 1976 Jun;6(3):265–269. doi: 10.1016/0020-7519(76)90044-8. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Williams J. F. The immunological response of the rat to infection with Taenia taeniaeformis. V. Sequence of appearance of protective immunoglobulins and the mechanism of action of 7Sgamma2a antibodies. Immunology. 1975 Nov;29(5):855–866. [PMC free article] [PubMed] [Google Scholar]
- NIETO D. Cysticercosis of the nervous system; diagnosis by means of the spinal fluid complement fixation test. Neurology. 1956 Oct;6(10):725–738. doi: 10.1212/wnl.6.10.725. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Wilson R. J. Evasion of the immune response by parasites. Br Med Bull. 1976 May;32(2):177–181. doi: 10.1093/oxfordjournals.bmb.a071352. [DOI] [PubMed] [Google Scholar]
- Powell S. J., Proctor E. M., Wilmot A. J., Barnett A. M. Neurological complications of cysticercosis in Africans: a clinical and serological study. Ann Trop Med Parasitol. 1966 Jun;60(2):159–164. doi: 10.1080/00034983.1966.11686400. [DOI] [PubMed] [Google Scholar]
- Powell S. J., Proctor E. M., Wilmot A. J., MacLeod I. N. Cysticercosis and epilepsy in Africans: a clinical and serological study. Ann Trop Med Parasitol. 1966 Jun;60(2):152–158. doi: 10.1080/00034983.1966.11686399. [DOI] [PubMed] [Google Scholar]
- Proctor E. M., Powell S. J., Elsdon-Dew R. The serological diagnosis of cysticercosis. Ann Trop Med Parasitol. 1966 Jun;60(2):146–151. doi: 10.1080/00034983.1966.11686398. [DOI] [PubMed] [Google Scholar]
- Rickard M. D., Outteridge P. M. Antibody and cell-mediated immunity in rabbits infected with the larval stages of Taenia pisiformis. Z Parasitenkd. 1974;44(3):187–201. doi: 10.1007/BF00328761. [DOI] [PubMed] [Google Scholar]
- Rosenberg E. B., Whalen G. E., Bennich H., Johansson S. G. Increased circulating IgE in a new parasitic disease--human intestinal capillariasis. N Engl J Med. 1970 Nov 19;283(21):1148–1149. doi: 10.1056/NEJM197011192832107. [DOI] [PubMed] [Google Scholar]
- Rydzewski A. K., Chisholm E. S., Kagan I. G. Comparison of serologic tests for human cysticercosis by indirect hemagglutination, indirect immunofluorescent antibody, and agar gel precipitin tests. J Parasitol. 1975 Feb;61(1):154–155. [PubMed] [Google Scholar]
- Schenone H., Aranda R., Concha L., Knierim F., Rojas A., Cofré H. Investigación de hidatidosis y cisticercosis inaparentes por medio de reacciones immunobiológicas. Bol Chil Parasitol. 1971 Jul-Dec;26(3):121–123. [PubMed] [Google Scholar]
- Somorin A. O., Heiner D. C., Ajugwo R. E. Immunoglobulin E in Nigerian onchocerciasis. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):872–876. doi: 10.4269/ajtmh.1977.26.872. [DOI] [PubMed] [Google Scholar]





