Abstract
We have used mice lacking both B7-1 and B7-2 costimulation molecules (B7KO) to investigate the effects of B7 costimulation on herpes simplex virus type 2 (HSV-2) pathogenesis. B7KO mice infected intravaginally with virulent HSV-2 showed more severe genital and neurologic disease and higher mortality rates than their wild-type counterparts. These results suggest that B7 costimulation molecules play an important role in the development of primary immune responses protective against HSV-2.
Activation and expansion of T cells is the central event in the generation of most adaptive immune responses. Naive T-cell activation depends upon two distinct stimuli. The first is the antigen-specific signal provided by interactions between the T-cell receptor and a processed antigenic peptide presented by a syngeneic major histocompatibility molecule. A second antigen-independent signal is mediated by the engagement of T-cell surface molecules with costimulatory molecules expressed by antigen-presenting cells (1, 2). The best-defined costimulatory molecules are B7-1 (CD80) and B7-2 (CD86). In mice, B7-2 is constitutively expressed on dendritic cells and macrophages and is upregulated on these and on B cells by activation (15). B7-1 is expressed at low levels only on dendritic cells but can be induced on B cells and macrophages by activation (3, 4, 7, 11, 12). The B7 costimulation molecules interact with CD28 and CTLA-4 molecules on the surface of the T cell. CD28 is constitutively expressed, and engagement with B7 molecules activates the T cell to proliferate and produce cytokines (10, 14). CTLA-4 expression on the T-cell surface occurs only after the cell becomes activated (8), and it appears to function as a negative regulator of T-cell activation (23).
Many in vitro and in vivo model systems have demonstrated the importance of B7 costimulation in the generation of antigen-specific immune responses. Costimulation also plays an important role in the induction of immune responses to most infectious agents. Transgenic expression of CTLA4Ig, a soluble fusion protein that selectively binds to B7 and blocks B7-ligand interactions, has been shown to hinder cytotoxic T-lymphocyte (CTL) responses to vesicular stomatitis virus (VSV) (24). Injection of mice with soluble CTLA4Ig decreases CTL expansion and effector activity against influenza virus (15) and HSV-1 (6). Class-switched immunoglobulin G (IgG) responses to influenza (15) and HSV-1 (9) are also diminished in the presence of soluble CTLA4Ig.
Germ line deletion of the genes encoding B7-1 and B7-2 represents a genetic approach to interruption of the B7 costimulation pathway that has the advantage of completely and selectively blocking B7-1 and B7-2 interactions with their ligands without affecting other potential costimulation partners of CD28 and CTLA-4. McAdam et al. (16) used mice deficient in B7-1 and B7-2 (B7KO) to demonstrate the dependence on B7 costimulation of the antigen-specific, CD8+ CTL response to VSV. Antibody responses to VSV in B7KO mice are also markedly altered. VSV replicates poorly and causes little pathology in the mouse, however, so the impact of these compromised immune responses on the capacity to resist pathogen-induced infection and disease could not be assessed.
Here we have used HSV-2 as a model system to investigate the influence of B7 costimulation on development of immune responses capable of controlling infection and disease. HSV-2 is a mucosal pathogen that spreads intraaxonally to the central nervous system. In a mouse model of primary genital infection with HSV-2, virus rapidly replicates to high titer in genital tissues and enters the nervous system, resulting in progressive paralysis and death. Given enough time after initiation of infection, the host will mount an immune response protective against severe pathology and death. HSV-specific T-cell subsets (20, 22, and L. A. Morrison, unpublished observations) and antibody (5, 21) both participate in immune-mediated recovery. We therefore have used low-dose HSV-2 infection of B7KO mice to assess the effect of B7 deficiency on the capacity to resist disease.
Mice genetically deficient in B7-1 and B7-2 (B7KO), crossed for 10 generations from the 129 strain onto a BALB/c background, and age-matched BALB/c mice were injected subcutaneously (s.c.) in the neck ruff with medroxy-progesterone (Depo-Provera; 3 mg/mouse in 100-μl volumes; Upjohn) 7 days and 1 day prior to infection. On the day of infection mice were anesthetized by intraperitoneal (i.p.) injection with sodium pentobarbital (1.6 mg/mouse), swabbed with a calcium alginate swab, and inoculated intravaginally (i.vag.) with a 5-μl volume containing either 500 (low dose) or 5,000 (high dose) PFU of HSV-2 G-6 (21). Acute virus replication in the genital tract and signs of genital and neurological disease were monitored over time postinfection.
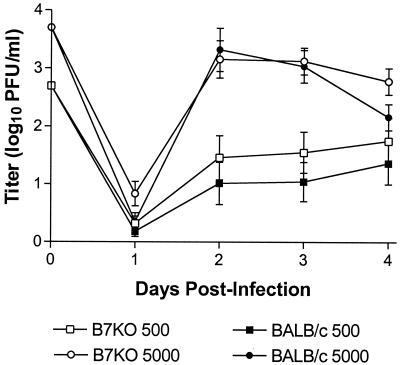
Virus shed from the genital epithelium was collected on days 1 through 4 postinfection and was quantitated by standard plaque assay (13). No significant differences in replication were observed between B7KO and BALB/c mice inoculated with either dose of virus (Fig. 1). This observation suggests that the presence or absence of B7 does not significantly alter responses within the genital mucosa during the first few days of primary infection.
FIG. 1.
Acute replication of virus in the genital tract. Groups of B7KO and BALB/c mice were inoculated i.vag. with HSV-2 G-6 at a dose of 500 or 5,000 PFU. Vaginal vaults were swabbed twice with calcium alginate swabs on days 1 through 4 postinoculation. Virus titer in swab samples was determined by standard plaque assay. Data from two experiments were pooled. Values represent the geometric mean titer ± standard errors of the means for 16 mice per group.
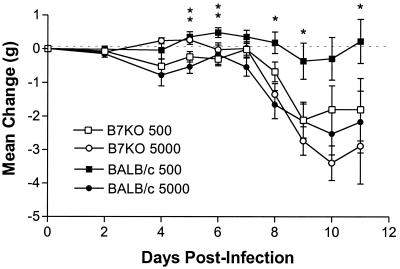
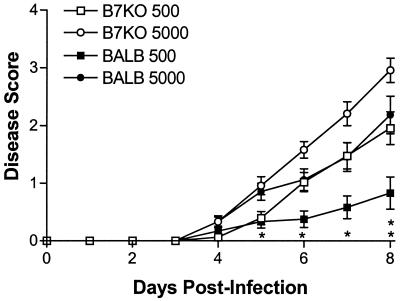
Mice were monitored daily after infection to assess body weight, signs of disease, and survival. Change in body weight, a sensitive indicator of the general health of the animal, was calculated based on comparison with preinfection body weight. B7KO mice lost considerably more weight than their BALB/c counterparts 7 to 10 days after i.vag. inoculation with the lower dose of HSV-2 (Fig. 2). In groups inoculated with the higher dose of virus, B7KO mice also lost more weight than BALB/c mice, but the difference was not statistically significant. B7KO mice receiving the lower dose of HSV also exhibited more severe genital and neurologic disease than BALB/c mice given the same dose of virus (Fig. 3). No difference in severity of disease was observed at the higher dose of HSV-2. The results of weight change and disease data indicate a dose-dependent deficiency in the ability of the B7KO mice to resist severe disease as a consequence of HSV-2 infection.
FIG. 2.
Change in body weight postinfection. Baseline weights of mice were obtained prior to infection, and mice were weighed each day following infection. Data from three experiments have been pooled, and values represent the mean weight change per group of 24 mice (8 mice per group per experiment). ∗, P ≤ 0.05; ∗∗, P ≤ 0.01.
FIG. 3.
Severity of disease postinfection. Mice were scored in masked fashion daily after infection according to the following scale: 0, no signs; 1, slight erythema and edema; 2, prominent erythema and edema; 3, presence of lesions; and 4, hind limb paralysis. Data from three experiments were pooled. Values represent the mean scores ± standard errors of the means for 24 mice per group. ∗, P ≤ 0.039; ∗∗, P ≤ 0.017.
In an initial experiment examining survival, we inoculated groups of B7KO and BALB/c mice with 50, 500, or 5,000 PFU of HSV-2 per mouse. All mice infected with the 50-PFU dose survived (Table 1), but the higher doses caused deaths, supporting the dose dependence of disease and survival phenotypes. All subsequent experiments were carried out at doses of 500 and 5,000 PFU/mouse. The results of four experiments, summarized in Table 1, demonstrate that a significantly greater proportion of the wild-type mice survived infection with 500 or 5,000 PFU of HSV-2 than the B7KO mice. In addition, B7KO mice succumbed to infection beginning approximately 1 day earlier than BALB/c mice receiving either dose of virus. A steep decline in survival occurred between days 9 and 12 in B7KO mice, while their wild-type counterparts exhibited a more gradual decline (data not shown).
TABLE 1.
Survival of B7KO mice and BALB/c mice after HSV-2 infection
| Dose (PFU) | Group | Proportion of mice surviving (no. surviving/no. infected)a |
|---|---|---|
| 50b | B7KO | 8/8 |
| 50 | BALB/c | 8/8 |
| 500c | B7KO | 9/32 |
| 500 | BALB/c | 23/32 (P = 0.0005) |
| 5,000c | B7KO | 5/31 |
| 5,000 | BALB/c | 15/31 (P = 0.0066) |
Values for B7KO mice were compared with values for BALB/c mice infected with the same dose of HSV-2 by the chi-square test.
Result of one experiment.
Pooled results of four experiments.
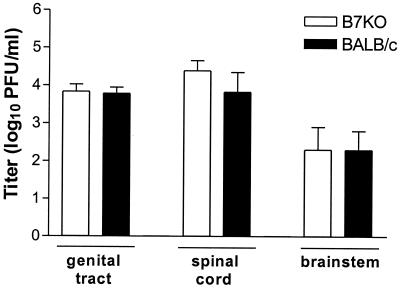
One possible explanation for the observed differences in disease and survival was a greater capacity of wild-type mice to inhibit virus spread to the genital tract parenchyma and the nervous system. To assess this, mice were infected with the low dose of HSV-2 and were sacrificed 3, 5, and 7 days postinfection. Genital epithelia were swabbed before tracts, spinal cords, and brainstems were dissected and assayed for virus. No significant differences in virus titers in genital tract tissue were observed between B7KO and BALB/c mice on days 3 and 5 postinfection, and no virus was detected in the spinal cords (data not shown). On day 7 postinfection there again was no difference between B7KO and BALB/c mice in virus titer in the genital tracts (Fig. 4). Robust replication of virus had occurred in the spinal cords and, to a lesser extent, the brainstems of both strains of mice. Although titer in the spinal cord was approximately fourfold higher in B7KO mice than in BALB/c mice, the difference was not statistically significant.
FIG. 4.
Virus replication in genital tract and nervous system tissues. Groups of mice were infected i.vag. with 500 PFU of HSV-2 G-6. Mice were sacrificed at days 3, 5, and 7 and genital tracts, spinal cords, and brainstems were dissected, homogenized, and assayed for virus replication. Data from two experiments were pooled. Values represent the geometric mean titers ± standard errors of the means for 10 mice per group.
Our results demonstrate that B7 costimulation is important in defense of the host against the pathological consequences of HSV-2 infection. Genital HSV-2 infection of mice lacking both B7-1 and B7-2 costimulatory molecules results in greater weight loss, more severe disease, and increased mortality compared to that of wild-type mice. This occurs even though HSV-2 replication at the primary site of infection and spread to and replication within the nervous system are not significantly different between the wild-type and B7KO mice at least through 7 days postinfection. Our results suggest that a lack of B7-costimulated immune responses in B7KO mice permits greater pathology to result from infection or that virus replication in the central nervous system is curtailed by immune responses late in infection of BALB/c but not of B7KO mice, permitting them to better avoid lethal neurologic injury.
Immune deficits in the B7KO mice that result in increased severity of disease after HSV infection could be several. Costimulation is critical in proper activation of T cells, and the absence of certain T-cell effector functions could render B7KO mice unable to effectively clear virus by conventional means such as CTL lysis. HSV-specific CTL are detected in genital lymph nodes between 4 and 8 days after infection, and in vitro depletion experiments demonstrated that antigen-specific lysis of HSV-infected target cells is predominantly mediated by CD4+ T cells (17). Existing reports indicate a marked inhibitory effect of B7 deficiency on generation of CD8+ T-cell-mediated responses to VSV (16). If generation of CD4+ CTL activity in the genital region in B7-deficient mice is similarly affected, it may influence disease phenotype, if not virus titer, during this time frame.
Decreased antiviral cytokine activity would also result from lack of proper T-cell activation in response to HSV infection. Mice treated with soluble CTLA4Ig to block B7-CD28 interactions prior to HSV infection fail to produce numbers of gamma interferon (IFN-γ)-producing CD4+ and CD8+ T cells equivalent to those produced by wild-type mice (6). Large numbers of IFN-γ-secreting cells are detectable in the spleen, genital lymph nodes, and genital tracts of wild-type mice by 4 to 5 days after vaginal HSV infection (17), and IFN-γ plays an important role in reduction of virus in the genital tract (19). The low dose of HSV-2 used in our experiments may delay stimulation of this potent HSV-specific T-cell activity in both wild-type and B7KO mice because viral titer in the genital tract increased through day 7 postinfection. The IFN-γ response may be further delayed or diminished in B7KO mice due to general failure to mount a normal, balanced T-cell response and may contribute to the increased susceptibility of B7-deficient mice to HSV-2-mediated genital disease. Experiments to test this possibility are in progress.
While IFN-γ is an important antiviral cytokine, IFN-γ produced by T-helper cells is also instrumental in driving certain class-switched antibody responses to infection. Plasma cells that secrete HSV-specific IgG are found in draining lymph nodes as early as day 7 after i.vag. infection (18), concurrent with the appearance of HSV-specific IgG and IgA in the serum (data not shown). However, HSV-specific antibody is not detected in vaginal wash fluid until 10 days postinfection (data not shown). Thus, antibody may not be available in time to aid in clearance of virus from the genital tract during primary infection (21), so it was not surprising that we observed no difference between B7KO mice and wild-type mice with regard to acute virus replication in the genital tract.
Passive transfer experiments performed by us and others (5, 21) have demonstrated a role for immune serum antibody in protection of the nervous system, and we did observe differences in the incidence of neurologic disease and mortality between B7KO and wild-type mice. Because HSV-specific antibody appears in the serum of wild-type mice by day 7 postinfection, the spread of virus to the spinal cord and brainstem may subsequently be reduced. Thus, if proper activation of T cells is prevented in the absence of B7, both the T-cell and B-cell arm of the immune response would be significantly compromised, and this may decrease the ability of B7KO mice to resist HSV-mediated pathology. We are presently addressing this possibility.
Acknowledgments
We thank Arlene Sharpe for the generous gift of the B7KO mice and Alex McAdam for helpful advice and discussions. We thank Li Zhu, John Patton, and Rob Reass for expert technical assistance and Lynn Dustin for careful review of the manuscript.
This work was supported by Public Health Service grant CA75052.
REFERENCES
- 1.Boussiotis, V., D. Barber, T. Nakarai, G. J. Freeman, J. Gribben, G. Bernstein, A. D'Andrea, J. Ritz, and L. M. Nadler. 1994. Prevention of anergy by signaling through the gamma c chain of the IL-2 receptor. Science 266:1039-1042. [DOI] [PubMed] [Google Scholar]
- 2.Boussiotis, V., J. Gribben, G. J. Freeman, and L. Nadler. 1994. Blockade of the CD28 costimulatory pathway: a means to induce tolerance. Curr. Opin. Immunol. 6:797-807. [DOI] [PubMed] [Google Scholar]
- 3.Boussiotis, V., G. J. Freeman, J. Gribben, J. Daley, G. Gray, and L. Nadler. 1993. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T cell activation. Proc. Natl. Acad. Sci. USA 90:11059-11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding, L., P. Linsley, L.-Y. Huang, R. Germain, and E. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the upregulation of B7 expression. J. Immunol. 151:1224-1234. [PubMed] [Google Scholar]
- 5.Dudley, K., N. Bourne, and G. N. Milligan. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270:454-463. [DOI] [PubMed] [Google Scholar]
- 6.Edelmann, K., and C. Wilson. 2001. Role of CD28/CD80-86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J. Virol. 75:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman, A., G. J. Freeman, K. Rhynhart, and L. Nadler. 1991. Selective induction of B7 BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell. Immunol. 137:429-437. [DOI] [PubMed] [Google Scholar]
- 8.Freeman, G. J., D. Lombard, C. Gimmi, S. Brod, K. Lee, J. Laning, D. Hafler, M. Dorf, G. Gray, H. Reiser, C. H. June, C. B. Thompson, and L. M. Nadler. 1992. CTLA-4 and CD28 mRNA are coexpressed in most T cells after activation. Expression of CTLA-4 and CD28 mRNA does not correlate with the pattern of lymphokine production. J. Immunol. 149:3795-3801. [PubMed] [Google Scholar]
- 9.Gangappa, S., E. Manickan, and B. T. Rouse. 1998. Control of herpetic stromal keratitis using CTLA4Ig fusion protein. Clin. Immunol. Immunopathol. 86:88-94. [DOI] [PubMed] [Google Scholar]
- 10.Gross, J., E. Callas, and J. Allison. 1992. Identification and distribution of the costimulatory receptor CD28 in the mouse. J. Immunol. 149:380-388. [PubMed] [Google Scholar]
- 11.Hathcock, K., G. Laszlo, C. Pucillo, P. Linsley, and R. Hodes. 1994. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J. Exp. Med. 180:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins, M. 1994. The ups and downs of T cell costimulation. Immunity 1:443-446. [DOI] [PubMed] [Google Scholar]
- 13.Knipe, D., M. Quinlan, and A. Spang. 1982. Characterization of two conformational forms of the major DNA-binding protein encoded by herpes simplex virus 1. J. Virol. 44:736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanier, L., S. O'Fallon, C. Somoza, J. Phillips, P. Linsley, K. Okumura, D. Ito, and M. Azuma. 1995. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 154:97-105. [PubMed] [Google Scholar]
- 15.Lumsden, J., J. Roberts, N. Harris, R. Peach, and F. Ronchese. 2000. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J. Immunol. 164:79-85. [DOI] [PubMed] [Google Scholar]
- 16.McAdam, A. J., E. Farkash, B. Gewurz, and A. H. Sharpe. 2000. B7 costimulation is critical for antibody class switching and CD8+ cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milligan, G. N., and D. I. Bernstein. 1995. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212:481-489. [DOI] [PubMed] [Google Scholar]
- 18.Milligan, G. N., and D. I. Bernstein. 1995. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology 206:234-241. [DOI] [PubMed] [Google Scholar]
- 19.Milligan, G. N., and D. I. Bernstein. 1997. Interferon gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 20.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 21.Morrison, L. A., L. Zhu, and L. G. Thebeau. 2001. Vaccine-induced serum immunoglobulin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J. Virol. 75:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parr, M. B., and E. L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walunas, T., D. Lenschow, C. Bakker, P. Linsley, G. J. Freeman, J. Green, C. Thompson, and J. Bluestone. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1:405-413. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann, C., P. Seiler, P. Lane, and R. M. Zinkernagel. 1997. Antiviral immune responses in CTLA4 transgenic mice. J. Virol. 71:1802-1807. [DOI] [PMC free article] [PubMed]